An investigation reveals significant discrepancies between SPF certifications from lab Princeton Consumer Research (PCR) and independent testing by consumer group Choice, raising serious questions about sunscreen efficacy and testing integrity. Experts flagged 'unusual' uniformity in PCR's human trial data, with major brands like Cancer Council, Woolworths, and Bondi Sands implicated. The findings threaten consumer trust in sun protection claims and highlight systemic oversight gaps.
Sunscreen SPF Testing Scandal: When Lab Certifications Don't Match Real-World Protection
A critical investigation into sunscreen efficacy has exposed potentially systemic flaws in the laboratory testing underpinning SPF (Sun Protection Factor) certifications for major brands sold in Australia. At the heart of the controversy is Princeton Consumer Research (PCR), an internationally accredited laboratory whose test results for numerous sunscreens diverged drastically from independent verification by consumer advocacy group Choice. The discrepancies raise profound concerns about the reliability of the regulatory framework ensuring sunscreens meet their advertised protection levels.
The Spark: Choice's Damning Findings
Choice's recent testing of 20 popular sunscreens branded as SPF 50+ delivered shocking results: 16 failed to meet their label claims. Ultra Violette's Lean Screen SPF50+ Mineral Mattifying Zinc Skinscreen recorded the lowest result – an SPF of just 4, a fraction of its claimed protection. Brands swiftly countered, pointing to their own lab certifications proving compliance. The ABC can now reveal that at least eight of these conflicting certifications originated from PCR, whose SPF results were consistently and significantly higher than Choice's findings.

Choice's testing found Ultra Violette's Lean Screen SPF50+ fell drastically short of its claimed protection. (ABC News: Billy Cooper)
Questioning the Data: Experts Raise Red Flags
The core issue lies in the methodology of SPF testing itself. Human trials involve exposing 10 volunteers to UV light with and without sunscreen, calculating individual SPF values, then averaging them for the final rating. Experts scrutinizing PCR's reports identified an alarming anomaly: unusually low variation between individual subject results.
Mathias Rohr, Chief Operating Officer of Germany's prestigious Normec Schrader Institute, reviewed PCR's Ultra Violette test data. "A table of results including only two different numbers is quite surprising for me," Dr. Rohr stated. "In my entire career, we have not [had] such homogeneous results in an in vivo SPF test." Normec Schrader later conducted a validation test for Choice on the same Ultra Violette product, confirming an SPF of 5.
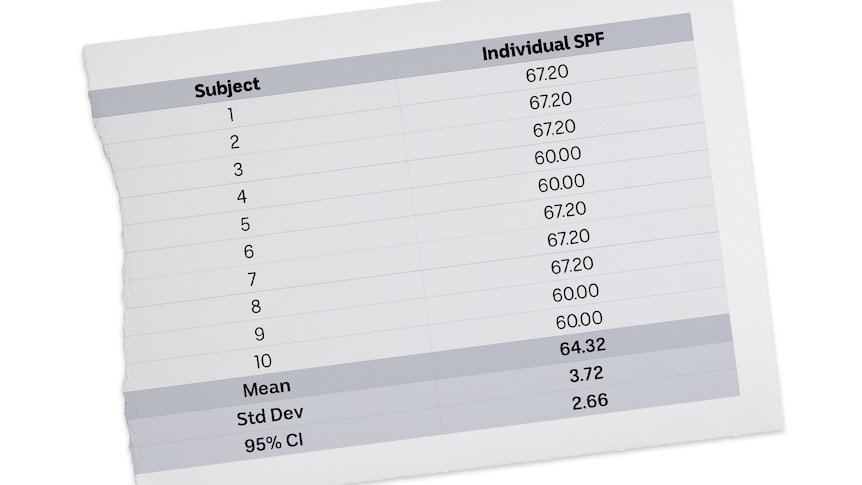
This recreation of PCR's test data for Ultra Violette shows minimal variation between subjects – described by experts as "unusual" and "surprising". (ABC News)
Other independent experts echoed these concerns. One senior industry scientist told the ABC the uniformity "would never happen in real life" and warranted investigation, while a statistician noted the frequency of identical results was "odd" and "unusual".
Major Brands Implicated, PCR Defends Methodology
The ABC confirmed PCR performed the original SPF validation for several sunscreens that underperformed in Choice's review, including:
- Three Cancer Council products
- Woolworths SPF 50+ Sensitive Sunscreen Lotion
- Coles SPF 50+ Sunscreen Lotion
- Ultra Violette Lean Screen SPF50+
- Bondi Sands SPF 50+ Fragrance Free Sunscreen
- Sun Bum SPF 50 Mineral Sunscreen Lotion
Cancer Council reports from PCR also showed minimal variation between test subjects – in one case, 9 out of 10 volunteers recorded identical SPF results. Cancer Council stated it was "investigating the matter thoroughly" and had submitted products to a different lab for retesting, while emphasizing the importance of continued sunscreen use. Woolworths, Coles, and Bondi Sands maintained their products met regulatory requirements based on their testing.
PCR Technical Director Barrie Drewitt defended the lab's work, calling the uniform results "uncommon" but "not inherently implausible, particularly with high-performing products and consistent application".

PCR Technical Director Barrie Drewitt defended the lab's results as "robust and verifiable". (Facebook: Princeton Consumer Research)
The ABC also reported that Drewitt was disqualified as a UK company director for eight years in 2010 related to the insolvency of a previous testing firm, Euroderm Research. While historical charges against Drewitt concerning fabricated clinical trial data at Euroderm were withdrawn by UK regulators in 2011 (with the judge directing not guilty verdicts), the background adds to scrutiny of PCR's current operations. Drewitt stated the past matters were legally resolved and had "no bearing" on PCR, emphasizing their methodologies are "robust and verifiable".
Regulatory Gaps and the Path Forward
The Therapeutic Goods Administration (TGA), Australia's sunscreen regulator, confirmed it is investigating Choice's findings but revealed a critical gap: it does not directly regulate third-party testing labs like PCR. It relies on manufacturers' self-certification that their products meet standards. The TGA stated it was recently made aware of Drewitt's past but reiterated sunscreen's vital role in sun safety alongside other protective measures.
Campbell Richards, CEO of major manufacturer Baxter Laboratories (which uses PCR), acknowledged the gravity of the situation: "Confidence in SPF testing is a priority... We recognise the significance of this moment for the category."
The Stakes: Trust in Sun Protection
This controversy transcends sunscreen brands; it strikes at the integrity of the certification process upon which consumers, especially in sun-drenched Australia, rely for skin cancer prevention. The significant discrepancies between PCR's certifications and independent verification, coupled with expert concerns over data plausibility, demand urgent, transparent investigation by regulators and the industry. The reliance on manufacturer-funded testing without robust, independent oversight of the labs themselves appears to be a critical vulnerability. Until resolved, the shadow over SPF labels leaves consumers questioning the reliability of a key public health tool.
Source: ABC News Investigation, July 2025

Comments
Please log in or register to join the discussion