A landmark Nature study reports that off-the-shelf SARS-CoV-2 mRNA vaccines can reprogram the immune system to make tumors more responsive to checkpoint inhibitors—without encoding any cancer antigens. For developers and biotech engineers behind mRNA platforms, the findings recast COVID-era tooling as a general-purpose immune orchestration layer for oncology.
When a COVID Shot Becomes Cancer Infrastructure
In one of the most surprising turns of the post-pandemic era, a new peer‑reviewed study in Nature reports that standard SARS-CoV-2 mRNA vaccines—designed only to encode viral spike protein—can make certain cancers significantly more responsive to immune checkpoint inhibitors (ICIs).
Not bespoke neoantigen vaccines. Not tumor-targeted constructs. The same architectural ideas and lipid nanoparticles that powered mass COVID-19 vaccination.
For technologists building mRNA platforms, immune-oncology pipelines, or LNP toolchains, this is more than a medical curiosity. It is a signal that our mRNA delivery infrastructure is itself a programmable, system-level modulator of human immunity—and we’ve only just started treating it that way.
Source: Grippin et al., “SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade”, Nature, 2025 (CC BY-NC-ND 4.0).
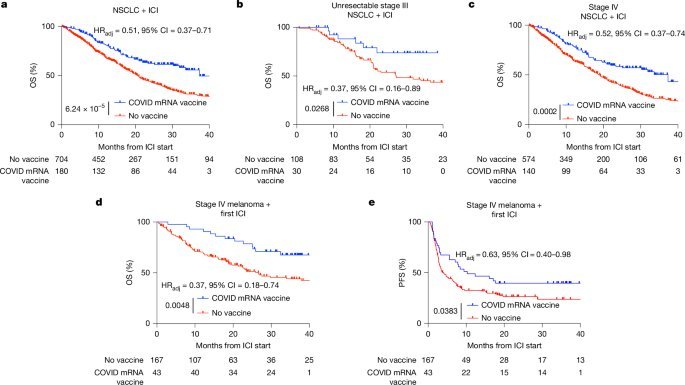
The headline result: a timing hack for survival
The MD Anderson–led team analyzed large retrospective cohorts of patients receiving ICIs for:
- Non-small cell lung cancer (NSCLC)
- Metastatic melanoma
- A broader, tissue-agnostic set of solid tumors
The key association: receiving a COVID-19 mRNA vaccine within roughly 100 days of ICI initiation correlated with markedly better outcomes.
Highlights:
- In NSCLC (n ≈ 884 ICI-treated):
- Patients vaccinated with a COVID-19 mRNA shot within 100 days of ICI start had a median overall survival of 37.3 months vs. 20.6 months for non-vaccinated peers.
- Adjusted hazard ratio ≈ 0.51 (p < 0.0001), robust after controlling for 39 covariates, immortal time bias, and propensity matching.
- In metastatic melanoma:
- Similar timing window → substantial gains in both overall and progression-free survival.
- Across a heterogeneous, tissue-agnostic ICI cohort:
- Any COVID-19 mRNA vaccination within 100 days of ICI initiation associated with significantly improved survival.
Control checks matter: influenza and pneumococcal vaccines in similar windows did not recapitulate the benefit. Nor did COVID-19 vaccination near chemotherapy in the absence of ICIs. The effect is specific to:
- mRNA/LNP-based vaccination, and
- its interaction with checkpoint blockade.
For practitioners and platform teams, this is the signal: The combination of mRNA vaccine–driven innate activation plus PD-1/PD-L1 inhibition is behaving like a composable system.
{{IMAGE:3}}
Mechanism as engineering: mRNA-LNP as an immune operating system
The group does what good systems engineers do: they don’t stop at correlation. They reconstruct, perturb, and profile the stack in mouse models and healthy humans.
Conceptually, the pipeline looks like this:
- Intramuscular mRNA-LNP injection
- A sharp, transient spike in type I interferons (especially IFN-α)
- Global activation of antigen-presenting cells (APCs) in lymphoid organs
- Enhanced presentation of tumor antigens
- Expansion and activation of tumor-reactive CD8+ T cells
- T-cell infiltration into tumors
- Tumor upregulation of PD-L1 (an adaptive resistance response)
- ICIs step in to block PD-1/PD-L1 and sustain the attack
This is, functionally, a form of remote, software-like reconfiguration of the tumor-immune interface using a standardized mRNA/LNP payload.
Preclinical reconstruction: BNT162b2, but as a probe
To test causality, the researchers synthesized mRNA and LNP formulations approximating Pfizer/BioNTech’s BNT162b2:
- Spike-encoding mRNA with N1-methyl-pseudouridine
- Encapsulated in clinically similar ionizable LNP compositions
- QC’d for size, polydispersity, charge, encapsulation efficiency, and antigenicity
In poorly immunogenic ("cold") murine tumor models (B16F0 melanoma, LLC lung cancer), spike mRNA-LNPs:
- Alone: modest or variable effects.
- With anti-PD-1/PD-L1: consistently superior tumor control and reduced metastases.
- Even when vaccination slightly precedes ICI, synergy persists.
Mechanistically:
- Blocking IL-1 signaling: no major impact.
- Blocking type I IFN signaling via IFNAR1: completely abrogates antitumor benefit.
- Direct high-dose IFN-α can partially mimic effects but lacks the spatial/temporal precision of LNP-driven activation.
In other words, it’s not just the encoded spike antigen. It’s the structured, LNP-mediated innate sensing event.
{{IMAGE:4}}
It’s the format, not the antigen
A critical systems insight for mRNA engineers:
- Swapping spike for an irrelevant antigen (CMV pp65 not expressed in the tumor) preserved antitumor effects.
- Enhancing innate visibility by replacing N1-methyl-pseudouridine with unmodified uridine in some constructs further boosted synergy with ICIs (though context-dependent).
- Removing trace dsRNA contaminants did not kill the effect; knocking out RIG-I did not eliminate IFN-α induction.
- LNP architecture mattered: alternative anionic assemblies (lipid particle aggregates) with the same mRNA did not reproduce the response.
The data support this model:
- LNP encapsulation plus higher-order mRNA structures likely engage MDA5 and related sensors.
- Result: a controlled type I IFN burst, tuned by particle design and mRNA chemistry.
For platform teams:
- The "vaccine" behaves like an innate immune middleware layer.
- Antigen choice is orthogonal; the core primitive is “inject LNP-mRNA, get a timed IFN-α + APC activation wave”.
- That wave can be layered with ICIs to flip tumors from tolerant to targetable.
Epitope spreading: from spike shot to tumor-directed T cells
Once the innate program is bootstrapped, the adaptive layer gets interesting.
In mice receiving spike mRNA-LNP + ICIs, the authors detect:
- Expansion of activated effector and effector-memory CD8+ T cells
- Strong PD-1 expression on these T cells, indicating active engagement
- Increased frequency of CD8+ T cells specific for classic melanoma-associated antigens (e.g., gp100, Trp2, survivin, WT1, CLDN6) via tetramer staining and activation-induced marker (AIM) assays
This is epitope spreading in action: a non-tumor antigen mRNA vaccine ignites innate immunity, which then:
- Enables APCs to more efficiently present endogenous tumor antigens in lymphoid tissues
- Seeds a more diverse, tumor-focused T-cell response that ICIs can protect from exhaustion
At the tumor site, they observe:
- A surge of PD-1+ CD8+ tumor-infiltrating lymphocytes
- Roughly 2x increase in tumor-reactive (tetramer+) CD8+ T cells among infiltrates with the combo regimen
- A pronounced upregulation of PD-L1 on tumor cells, tightly linked to type I IFN signaling
{{IMAGE:5}}
For developers of checkpoint regimens, this is the operational takeaway: mRNA-LNPs can be used to:
- Increase the dynamic range of PD-1/PD-L1 biology in tumors
- Ensure there is “something to block” with ICIs, especially in tumors that start cold
Human data: the IFN-α burst is real
To validate that this machinery isn’t mouse-only, the team profiled healthy volunteers given:
- Moderna’s mRNA-1273 (50 µg, 2023–24 XBB.1.5 formulation)
- Pfizer/BioNTech’s BNT162b2 (30 µg, 2024–25 formulation)
Key observations:
- IFN-α is one of the most strongly induced cytokines ~24 hours post-vaccination.
- Levels jump ~280x over baseline (into low pg/mL) in the mRNA-1273 cohort; induction is more modest but qualitatively similar with BNT162b2.
- At 24 hours, a broader inflammatory signature appears (IFN-γ, IFN-ω, CXCL10, IL1RN, etc.), then normalizes by day 7.
- Flow cytometry shows transient:
- PD-L1 upregulation on circulating myeloid cells and dendritic cells
- Activation markers on NK cells
- Early T-cell activation (CD69 upregulation)
The timing and structure of this human response line up strikingly well with the murine mechanism: a fast, programmable, non-permanent innate surge.
PD-L1 expression: vaccines nudging clinical thresholds
One of the more pragmatic—and provocative—findings sits at the diagnostic boundary.
In >2,300 NSCLC biopsy reports with PD-L1 tumor proportion scores (TPS), the authors compared PD-L1 levels by proximity to COVID-19 mRNA vaccination:
- Vaccinated within 100 days before biopsy:
- Mean TPS ≈ 31%
- No prior COVID-19 mRNA vaccine:
- Mean TPS ≈ 25%
- Vaccinated ≥100 days before biopsy:
- Mean TPS ≈ 22%
Patients vaccinated within 100 days were also more likely to cross the pivotal TPS ≥50% threshold that guides eligibility for ICI monotherapy vs. chemo-immunotherapy.
Influenza and pneumococcal shots showed no such association.
Extended to a broader, tissue-agnostic cohort (>5,000 PD-L1–stained samples), the same pattern emerges: recent mRNA vaccination modestly but significantly elevates PD-L1 TPS.
This directly ties the mechanistic story to real-world decision logic:
- The immunologic “wake-up call” from an mRNA vaccine can temporarily shift a tumor’s biomarker profile into a range where ICIs are both biologically and regulatorily favored.
The most important twist: rescuing cold tumors
For immunologists and clinical AI modelers who’ve long treated PD-L1-low tumors as poor candidates, the crucial result is this:
- Among stage IV NSCLC patients with baseline PD-L1 TPS <1% (classic “cold” tumors):
- Those who received a COVID-19 mRNA vaccine within 100 days of ICI initiation achieved survival outcomes approaching those of patients whose tumors were PD-L1 positive at baseline.
The association between vaccination and improved survival held across TPS strata (<1%, 1–49.9%, ≥50%), and was not simply an artifact of pandemic-era treatment changes.
Interpreted as a system:
- mRNA/LNP + ICI turns "cold" into "addressable".
- Off-the-shelf vaccination behaves like an on-demand preconditioning job for the immune system, scheduled around checkpoint therapy.
Why this matters to builders of mRNA, LNPs, and oncology platforms
For a technically literate audience, this paper is less "COVID vaccines cure cancer" and more "we’ve discovered a general-purpose immune modulation primitive hiding in plain sight." Several implications follow.
1. mRNA-LNP as a reusable immunologic runtime
The study reinforces that:
- The key abstraction is not "vaccine against X", but "structured mRNA + LNP event" that:
- Triggers a quantifiable, time-bounded type I IFN program
- Rewires APC behavior across lymphoid and tumor sites
- Is tunable via mRNA chemistry, dose, and LNP composition
For platform teams, this looks like a runtime on top of which you can:
- Layer tumor-targeting antigens (personalized or shared)
- Stack ICIs, bispecifics, or cell therapies
- Or, as this study shows, even exploit non-tumor antigens when time or manufacturing capacity is constrained
2. From bespoke neoantigens to off-the-shelf immune bootloaders
Personalized mRNA cancer vaccines are powerful but slow and expensive to manufacture. This work suggests:
- Off-the-shelf mRNA-LNP constructs—potentially encoding infectious disease antigens—can serve as a "bootloader" that:
- Primes systemic immunity
- Supports epitope spreading to native tumor antigens
- Sensitizes tumors so that ICIs and other modalities have something to engage
That’s a radically different cost and logistics profile, especially for health systems without deep personalized manufacturing pipelines.
3. Designing next-gen LNPs with intentional innate signatures
The mechanistic details should directly influence LNP and mRNA stack design:
- MDA5 vs RIG-I engagement depends on RNA structure and formulation.
- N1-methyl-pseudouridine dampens innate sensing, but carefully re-introducing visibility (e.g., partial uridine, structural motifs) can be leveraged in oncology.
- LNP charge, composition, and microstructure are not neutral—they define which pattern recognition receptors fire, when, and where.
Future platforms can:
- Offer "profiles" (e.g., antiviral-like IFN burst vs. quieter delivery) selectable per indication.
- Provide programmable innate footprints as a first-class API surface.
4. Rethinking clinical workflows and AI triage tools
If validated in prospective trials (a critical caveat), timing of mRNA vaccination may become a controllable parameter in:
- ICI treatment planning
- Clinical decision-support models
- Trial design and stratification algorithms
For data teams, there’s an uncomfortable but necessary realization:
- Models trained on pre-2020 immunotherapy outcomes may be miscalibrated for a world where many patients receive mRNA vaccines proximal to ICI.
- PD-L1 measurements—and downstream policy—might be influenced by recent mRNA exposures.
Accounting for "mRNA vaccination as a latent covariate" will not be optional.
A new kind of infrastructure moment
The COVID-19 vaccination campaign forced biotech to industrialize mRNA manufacturing, LNP formulation, and cold-chain distribution at planetary scale. At the time, it looked like a heroic one-off.
This Nature paper reframes it as something closer to the early internet: a crisis-driven deployment of infrastructure that turns out to support far more than its original use case.
We now have:
- Validated, mass-produced mRNA-LNP frameworks.
- A mechanistic demonstration that these frameworks can act as modular immune recalibrators.
- Early clinical evidence that, when composed with checkpoint inhibitors, this recalibration can turn previously unresponsive tumors into survivors.
For developers, engineers, and tech leaders in biotech, the mandate is clear:
- Treat mRNA-LNP not only as content (what antigen do we encode?) but as protocol (how do we shape systemic immunity?).
- Design platforms where innate activation profiles are as tunable as coding regions.
- Build clinical and data systems that understand vaccination events as interventions in the same graph as drugs and diagnostics.
Because the quiet lesson of this work is not that "a COVID shot fights cancer"—it’s that a well-designed packet of mRNA and lipids can act as an upgrade to the immune system’s runtime environment. And in oncology, as in computing, the biggest breakthroughs often come when we realize the infrastructure we already deployed can do more than we ever asked of it.
Comments
Please log in or register to join the discussion