Researchers unveil a-Heal, a closed-loop bioelectronic system combining wearable sensors, automated drug delivery, and ML-driven diagnostics to dynamically optimize wound healing. Early large-animal trials show accelerated tissue regeneration and reduced inflammation, signaling a paradigm shift in personalized care.
Chronic wounds affect millions globally, often leading to severe complications when timely medical intervention is inaccessible. Traditional static treatment protocols struggle to adapt to the dynamic biological processes of wound healing. Now, a breakthrough platform named a-Heal promises to revolutionize care through real-time diagnostics and adaptive therapy.
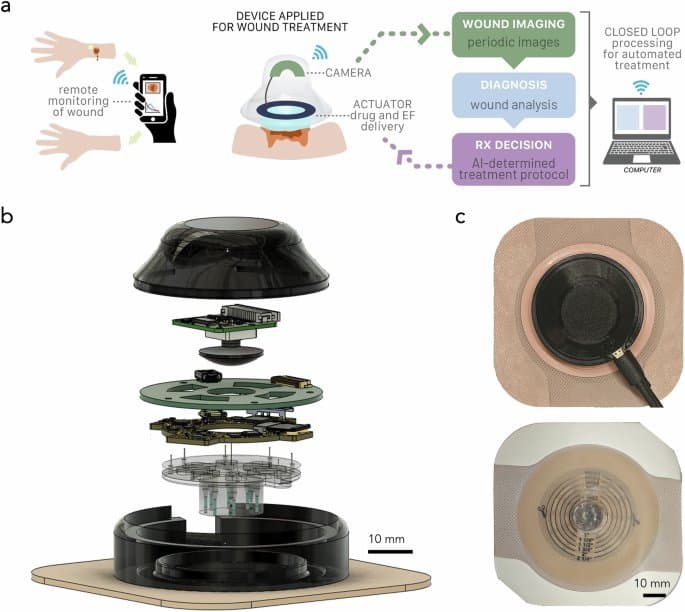
The a-Heal Architecture: Closed-Loop Healing
The system comprises two tightly integrated components:
The Wearable Device: A 3D-printed, waterproof unit housing:
- An imaging module with a camera, plano-convex lens, and LED ring for high-resolution wound capture every 2 hours.
- Bioelectronic actuators featuring an 8-channel ring PCB and iontophoretic pump body with reservoirs. These deliver precise doses of therapeutics (like fluoxetine) or apply controlled electric fields (EF up to 400 mV/cm), guided by COMSOL-optimized simulations.
- A microcontroller (ESP32-PICO-D4) handling wireless communication (Wi-Fi), sensor data processing (via onboard ADCs/DACs), and actuator control.
The ML Physician: An AI engine performing closed-loop control:
- Deep Mapper: An autoencoder converts wound images into a 4-state vector (hemostasis, inflammation, proliferation, maturation). It learns linear dynamics (
z_{k+1} = A z_k) to model healing stage transitions. - Optimal Control: Uses Linear Quadratic Regulator (LQR) theory (
v_k = -K z_k) to derive an ideal healing trajectory minimizing closure time. - Deep Reinforcement Learning (DRL) Controller: Two "follower" agents (for EF intensity and drug dosage) use an Actor-Critic algorithm. Their reward function (
r = exp(-η ||h^{-1}(z*_{k+1}) - x_{k+1}||_2) minimizes deviation from the LQR-projected wound state image, dynamically adjusting therapy.
- Deep Mapper: An autoencoder converts wound images into a 4-state vector (hemostasis, inflammation, proliferation, maturation). It learns linear dynamics (
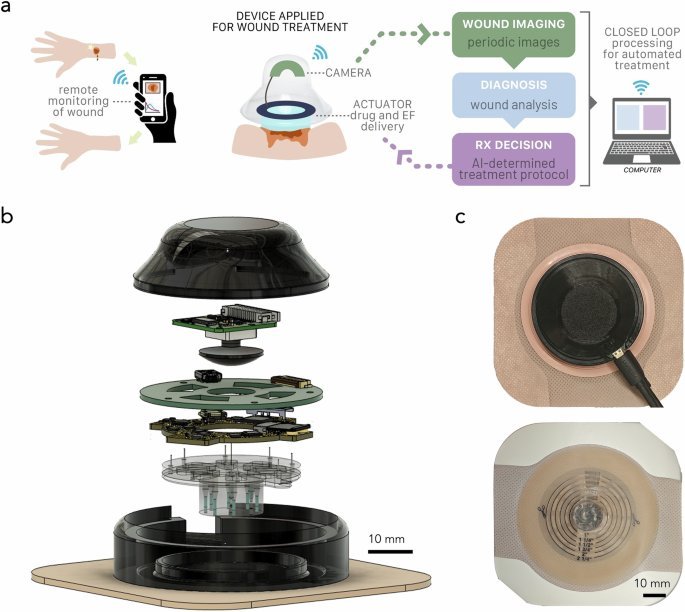 The a-Heal system integrates imaging, ML diagnostics, and bioelectronic therapy delivery in a closed loop.
The a-Heal system integrates imaging, ML diagnostics, and bioelectronic therapy delivery in a closed loop.
Validation: Accelerating Healing in Porcine Models
In a 22-day study on porcine excisional wounds:
- Closed-Loop Operation (Days 0-7): The system captured images, diagnosed stages, and delivered EF therapy until inflammation peaked (~40% probability), then transitioned to fluoxetine delivery.
- Results (Day 22):
- 34.2% thicker epidermis in treated wounds.
- 26.3% reduction in granulation tissue area.
- Improved collagen maturity (lower Type III/I ratio).
- Modulated inflammation: 61% reduction in IL1B, 39% increase in anti-inflammatory IL10.
- Higher re-epithelialization rates (51.8% vs 15.0% in controls).
- Device failures interrupting therapy resulted in poorer outcomes, underscoring the algorithm's impact.
 Histological analysis revealed significant improvements in tissue structure and reduced inflammation with a-Heal treatment.
Histological analysis revealed significant improvements in tissue structure and reduced inflammation with a-Heal treatment.
Engineering Innovations & Challenges
- Optical Clarity: Custom PDMS molding ensured clear imaging paths through the device.
- Precision Delivery: Iontophoretic pumps with hydrogel-filled capillaries enabled controlled drug release, calibrated via HPLC.
- Power Management: A 2-hour sleep cycle between imaging extended battery life to 24 hours.
- Limitations: Sample size was modest; tested only on non-infected wounds; fluoxetine isn't FDA-approved for wounds; device size needs miniaturization for complex wounds.
Implications: Beyond the Bandage
a-Heal represents a leap toward autonomous medical devices. By merging real-time biosensing, adaptive ML control, and targeted bioelectronic intervention, it shifts wound care from reactive protocols to proactive, personalized healing. This framework isn't confined to wounds—it signals a future where embedded AI dynamically manages chronic conditions, potentially democratizing access to precision medicine in resource-limited settings.
Source: Li, H. et al. Towards adaptive bioelectronic wound therapy with integrated real-time diagnostics and machine learning–driven closed-loop control. npj Biomed. Innov. 2, 31 (2025).
Comments
Please log in or register to join the discussion