Groundbreaking research maps how genes linked to autism, schizophrenia, and cortical malformations become active during critical early phases of human brain development using stem cell models and computational analysis. The study reveals disorder-specific 'critical phases' in neural stem cell development and predicts the impact of gene disruptions, opening new paths for targeted interventions.
For decades, the origins of complex neurodevelopmental and psychiatric disorders like autism spectrum disorder (ASD), schizophrenia (SCZ), and cortical malformations have been traced primarily to dysfunctions in mature neurons. However, a pivotal study published in Nature Communications reveals that the roots of these conditions may lie much earlier—in the very architects of the brain: neural stem cells (NSCs).
Modeling the Invisible Phase of Brain Development
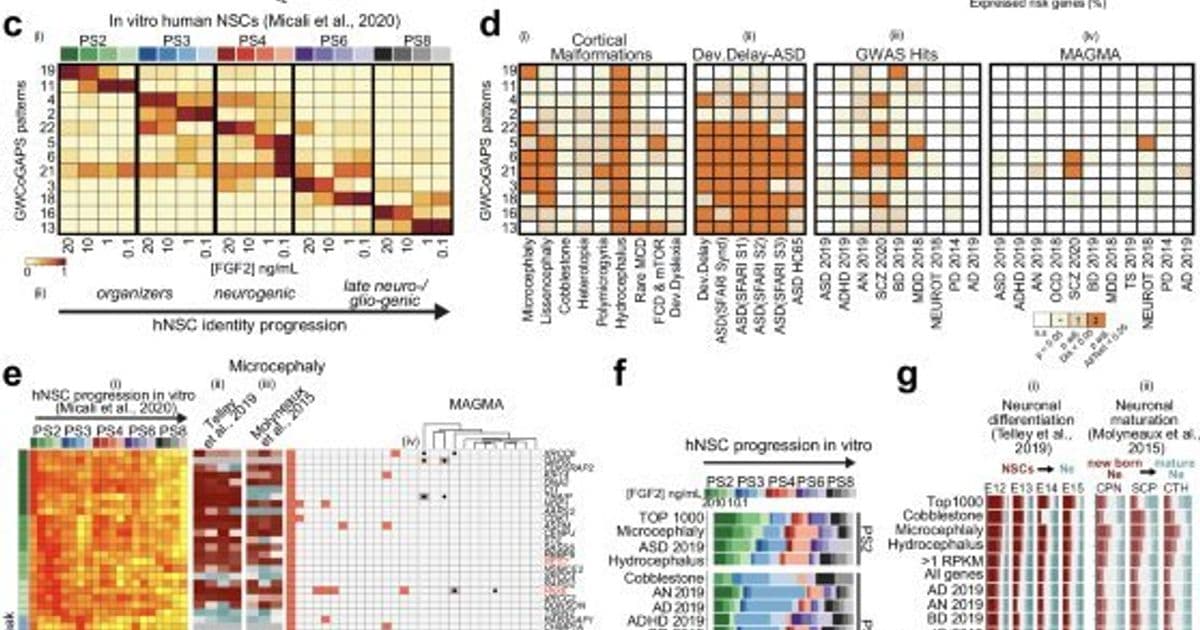
Researchers employed a powerful combination of human induced pluripotent stem cell (hiPSC)-derived models, single-cell genomics, and computational simulations to dissect the earliest stages of telencephalic development—the brain region giving rise to the cerebral cortex. Their in vitro NSC system recapitulated key developmental transitions:
- Early neuroepithelial/organizer progenitors (resembling brain patterning centers)
- Mid-passage neurogenic radial glia (generating neurons)
- Late neuro/gliogenic radial glia (generating glia)
By analyzing bulk and single-cell RNA-seq data across these stages, alongside primary human and cross-species (macaque, mouse) fetal brain datasets, the team mapped the expression dynamics of over 2,800 genes associated with 25 cortical malformations (like microcephaly, lissencephaly) and neuropsychiatric disorders (ASD, SCZ, ADHD).
Identifying Disorder-Specific Critical Phases
The analysis revealed distinct critical phases during NSC progression when genes linked to specific disorders are highly active and potentially most vulnerable to dysfunction:
- Early Phases (Neuroepithelial/Organizer): Microcephaly (MIC) and Hydrocephalus (HC) risk genes (e.g., ASPM, CENPJ, ARX) peaked early, suggesting disrupted patterning and cell cycle in founder cells.
- Mid Neurogenesis: Lissencephaly (LIS) and Schizophrenia (SCZ) genes (e.g., DCX) showed high expression, implicating neuronal fate commitment.
- Late Neuro/Gliogenesis: Focal Cortical Dysplasia (FCD) and mTORopathy genes (e.g., mTOR, DEPTOR) were prominent, aligning with known roles in late development.
"We identified vulnerability windows—specific NSC states during human telencephalic development—where dysfunction of risk genes is most likely to initiate a pathological cascade leading to distinct disorders," the researchers noted, emphasizing a paradigm shift towards early developmental origins.
Regulatory Networks and In Silico Perturbations
Using RcisTarget, the team constructed disease-specific gene regulatory networks (regulons), uncovering transcription factor (TF) hubs like KLF4 and MEF2C that orchestrate risk gene expression across disorders. To predict functional impacts, they leveraged CellOracle on human fetal cortex single-cell multi-omics data:
* Simulated knockouts (KOs) of disease-associated TFs (e.g., KLF6, MEF2C, ARX) revealed **spatiotemporal-dependent effects** on cell fate trajectories.
* Perturbations altered transitions between ventricular radial glia (vRG), outer radial glia (oRG), intermediate progenitors (IPCs), and neurons.
* KLF family TFs emerged as central hubs, suggesting their disruption could have broad consequences across corticogenesis.
This computational approach pinpointed "risk spatiotemporal-domains" where specific TF disruptions disproportionately impact neural lineage development.
Patient-Specific Insights in Autism
Analyzing NSCs derived from idiopathic ASD patients revealed recurrent dysregulation:
- Altered Patterning: Downregulation of dorsoposterior (DP) identity genes (WLS, EMX2, FEZF2) and upregulation of anteroventral (AV) genes (FOXG1, MEIS2) in early NSCs.
- Dysregulated Fate Regulators: Key TFs like POU3F2, LHX2, HES1, and YBX3 showed significant expression changes across multiple ASD donors.
- Cell Cycle Shifts: ASD-derived NSCs exhibited a higher proportion of cells in G1 phase, hinting at altered neurogenic commitment.
Comparison with a larger ASD organoid dataset confirmed frequent dysregulation of these early NSC regulators, particularly at the initial differentiation stage (TD0).
Implications and a New Resource
This work fundamentally shifts the focus to very early NSC phases and telencephalic patterning as critical etiological periods for diverse cortical disorders. It provides:
- A Framework for Precision Medicine: Identifying individual-specific critical phases and TF disruptions could guide targeted interventions.
- Computational Tools: The integrated use of stem cell models, single-cell genomics, and CellOracle simulations offers a blueprint for modeling developmental disorders.
- NeMO Analytics Resource: All data is available via NeMO Analytics, enabling exploration of risk gene expression across development stages and species.
The discovery of recurrent NSC and patterning defects in ASD patient lines, even without known high-risk mutations, underscores the power of this approach to uncover hidden drivers of complex disorders. By decoding the molecular blueprint laid down in the earliest days of brain formation, this research illuminates new paths for understanding and ultimately preventing neurodevelopmental and psychiatric diseases.
Source: Mato-Blanco, X. et al. Early developmental origins of cortical disorders modeled in human neural stem cells. Nat Commun 16, 6347 (2025). https://doi.org/10.1038/s41467-025-61316-w
Comments
Please log in or register to join the discussion