Withings' latest innovation, the BeamO smart thermometer, has received FDA clearance, offering more than just temperature checks by monitoring heart and lung health in under a minute. This device integrates seamlessly with Withings' ecosystem, empowering users with actionable health insights typically reserved for clinical settings. As wearable health tech evolves, BeamO signals a shift toward proactive, at-home vital signs tracking.
Withings BeamO: The FDA-Cleared Smart Thermometer Redefining Home Health Monitoring
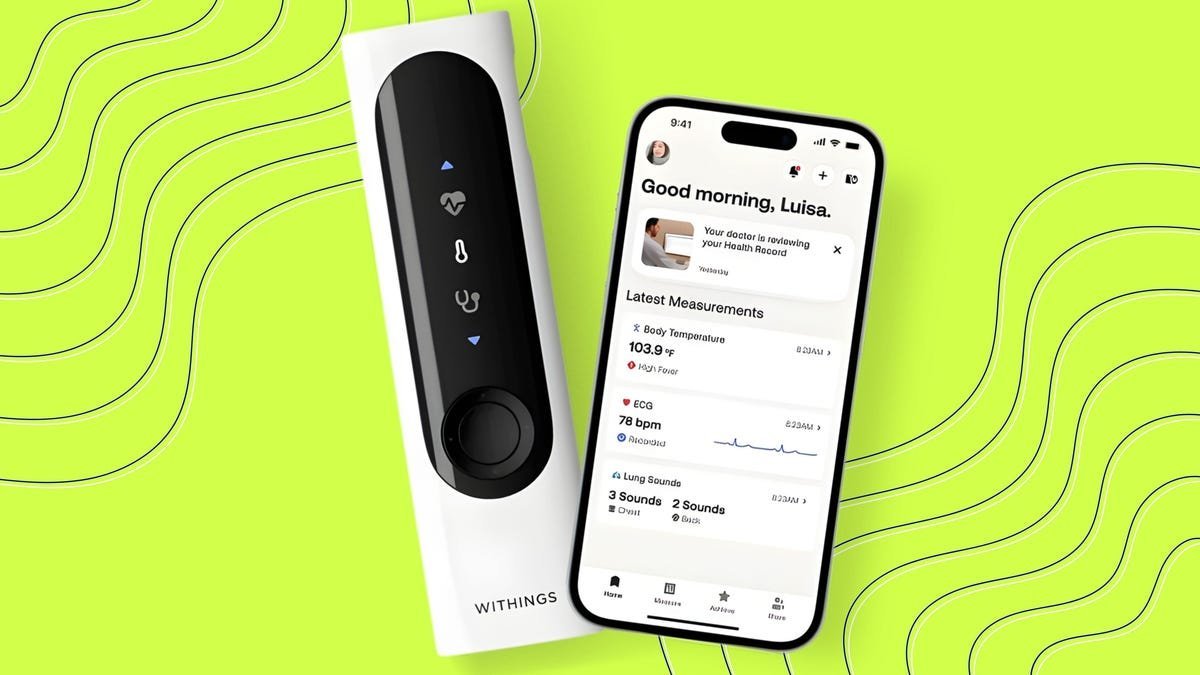
In an era where health monitoring is increasingly moving from clinics to the palm of your hand, Withings has unveiled a game-changer. The BeamO, a multifunctional smart thermometer, received FDA clearance on Thursday, November 13, 2025. This compact device doesn't just measure body temperature—it's a comprehensive health screening tool that captures electrocardiogram (ECG) data, monitors lung sounds, and provides insights into cardiac and pulmonary health, all in less than a minute. For developers and engineers in the health tech space, this launch underscores the growing intersection of consumer electronics, AI-driven analytics, and regulatory-approved medical devices.
From Mercury to Multisensor: The Evolution of Thermometers
Traditional thermometers have come a long way from their mercury-filled days. Today's smart devices leverage infrared sensors, acoustic wave detection, and electrical activity monitoring to deliver a holistic view of wellness. The BeamO exemplifies this evolution, using its built-in sensors to record heart electrical activity, infrared light for temperature, and acoustic waves for heart and lung interpretation. This data is then processed through the Withings app, where AI algorithms detect deviations from a user's personal baseline, offering personalized health trends without the need for a doctor's visit.
As Eric Carreel, Withings' founder and president, emphasized in the company's press release, "BeamO brings access to key vital signs, typically measured during medical consultations, into everyday life." This statement highlights the device's potential to democratize health data, especially during peak seasons for illnesses like flu or respiratory infections. For tech professionals building IoT health ecosystems, BeamO's capabilities demonstrate how edge computing and real-time data processing can enhance user engagement while maintaining privacy through app-based analysis.
Integration and AI: Powering Proactive Health Insights
What sets BeamO apart is its seamless integration with Withings' broader ecosystem. The device connects to HealthLink, a feature in the Withings app that aggregates data from compatible wearables and generates shareable reports for clinicians. This is particularly valuable for developers working on API-driven health platforms, as it showcases standardized data exchange protocols that could inspire interoperable solutions in telemedicine.
Additionally, Withings+ subscribers gain access to Cardio Check-Up, a service providing board-certified cardiologist reviews within 24 hours. While BeamO itself doesn't diagnose conditions, its ability to track lung sounds and temperature over time can aid doctors in assessing respiratory issues more effectively. Imagine the implications for AI/ML engineers: training models on longitudinal health data from devices like BeamO could lead to predictive algorithms for early illness detection, reducing the burden on healthcare systems.
Earlier this year, Withings introduced features in its smartwatches that alert users to potential sickness, building on this trend of anticipatory health tech. BeamO extends that philosophy, turning a simple thermometer into a frontline tool for monitoring during vulnerable periods, such as winter viral outbreaks.
Availability and Regulatory Milestone
Priced accessibly for early adopters, BeamO is now available directly from Withings' website, with a complimentary one-month Withings+ subscription and a free Cardio Check-Up included. It will expand to retailers like Amazon in 2026. The FDA clearance is a significant milestone, confirming that BeamO is "substantially equivalent" to existing medical devices, as per FDA standards. This approval not only validates Withings' engineering rigor but also paves the way for similar innovations in consumer health tech.
For engineers and developers, the BeamO's journey from prototype to cleared device illustrates the challenges of navigating regulatory landscapes while pushing technological boundaries. It's a reminder that robust sensor fusion and AI interpretation are key to creating trusted, user-centric health tools.
As we look ahead, devices like BeamO are blurring the lines between consumer gadgets and medical instruments, fostering a future where health monitoring is as routine as checking your smartphone. This shift not only empowers individuals with data-driven decisions but also challenges the tech industry to innovate responsibly, ensuring accuracy and accessibility for all.
Comments
Please log in or register to join the discussion