University of Minnesota researchers have pioneered a hybrid approach combining 3D-printed scaffolds, stem cells, and neural organoids to bypass spinal cord injuries in rats. The technique directed new nerve growth across severed spinal regions, enabling significant functional recovery—offering a transformative blueprint for future human treatments.
For over 300,000 Americans living with spinal cord injuries, the inability of severed nerves to regenerate has meant irreversible paralysis. Now, a breakthrough from the University of Minnesota delivers radical hope: rats with completely severed spinal cords regained mobility after implantation of a 3D-printed "neural relay" system. Published in Advanced Healthcare Materials, the research merges precision engineering, stem cell biology, and organoid science into a single therapeutic strategy.
Engineering a Neural Bypass
At the core of the innovation is a microscale 3D-printed scaffold, designed with intricate channels that act as guide rails for neural growth. Researchers populated these biocompatible structures with spinal neural progenitor cells (sNPCs) derived from human adult stem cells. Unlike previous attempts, the scaffold doesn’t just support cells—it actively directs their development.
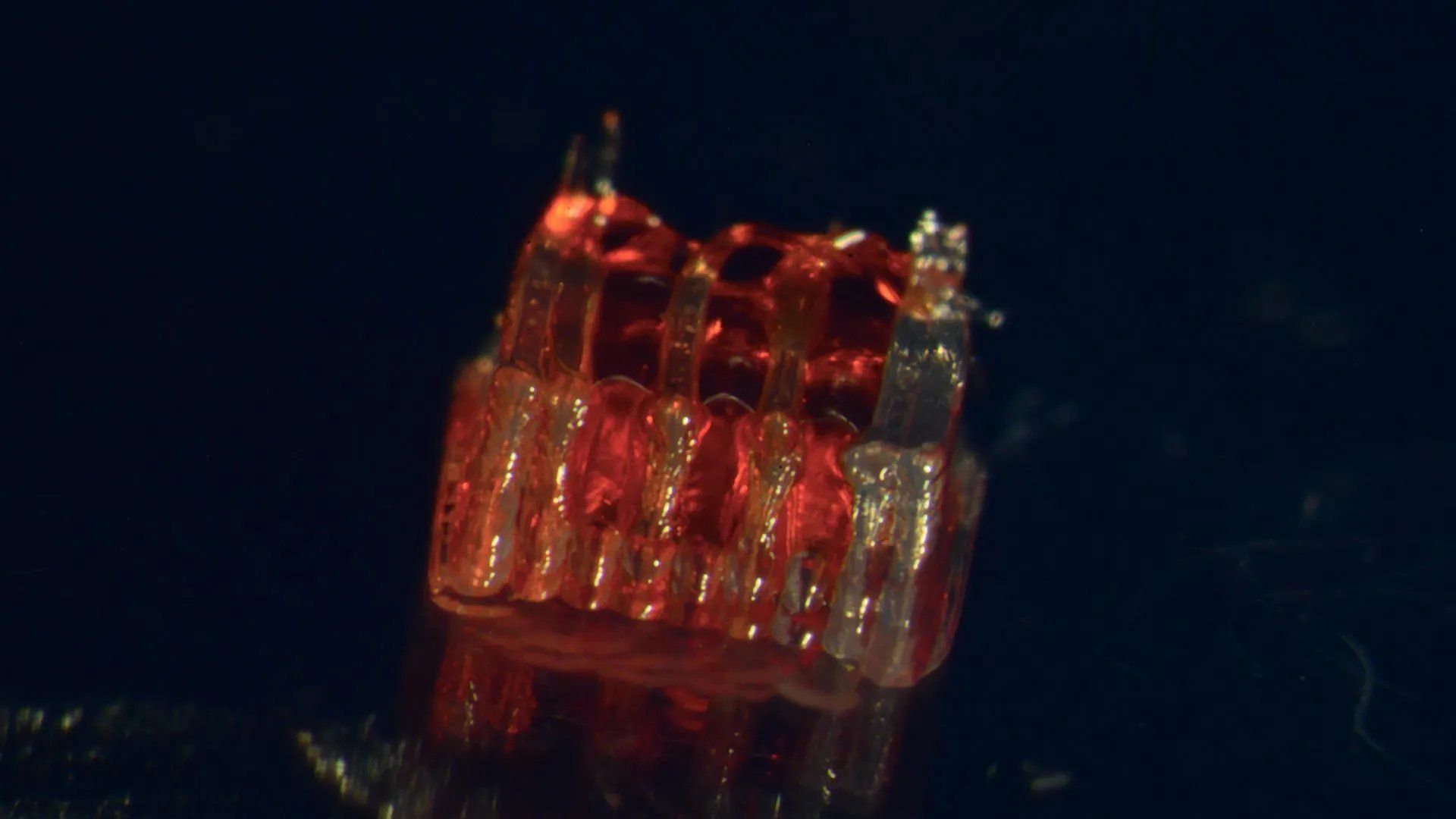 The 3D-printed scaffold features microchannels that organize growing nerve cells into functional pathways. (Credit: McAlpine Research Group, University of Minnesota)
The 3D-printed scaffold features microchannels that organize growing nerve cells into functional pathways. (Credit: McAlpine Research Group, University of Minnesota)
"We use the 3D-printed channels to dictate the growth trajectory of stem cells," explained lead author Guebum Han. "This creates a biological relay that bridges the injury site, rerouting neural signals around the damage."
When implanted into rats with fully transected spinal cords, the sNPCs within the scaffold differentiated into mature neurons. Crucially, these new nerve cells extended fibers rostral and caudal to the injury, integrating with the host’s existing neural circuitry. Over time, this rebuilt connection led to measurable restoration of motor function.
Why This Approach Breaks New Ground
- Directional Precision: The scaffold’s microchannels solve a critical challenge in neural repair—ensuring axons grow in structurally and functionally aligned pathways.
- Human Cell Compatibility: Using adult stem cell-derived sNPCs avoids ethical concerns and enhances potential for human translation.
- Integration Speed: New neurons formed synaptic connections with host tissue faster than natural regeneration attempts, accelerating functional recovery.
The Road to Clinical Impact
While still experimental, the technique represents a paradigm shift. Current spinal injury treatments focus on rehabilitation or rudimentary nerve grafting, neither of which rebuilds complex neural circuits. This method engineers a living, integrated repair system.
"Regenerative medicine has ushered in a new era for spinal cord research," said neurosurgery professor Ann Parr. "Our 'mini spinal cords' offer a scaffold not just for cells, but for hope."
Challenges remain, including scaling scaffold production and ensuring long-term stability in humans. Yet with funding from the NIH and Minnesota’s spinal injury research programs, the team is optimizing the platform. The convergence of bioprinting, stem cell programming, and microengineering showcased here may soon move from rat models to clinical trials—potentially rewriting futures for those told recovery is impossible.

Comments
Please log in or register to join the discussion