New research from St Bartholomew's Hospital demonstrates Apple Watch's effectiveness in detecting atrial fibrillation recurrences after ablation procedures, with earlier detection and fewer hospitalizations.
A groundbreaking clinical trial from St Bartholomew's Hospital in London has demonstrated that Apple Watch can significantly improve post-ablation monitoring for atrial fibrillation patients, potentially changing how doctors track heart rhythm after treatment. The study, published in the Journal of the American College of Cardiology, compared traditional clinic-based follow-up with a patient-led Apple Watch ECG monitoring program.
The research focused on patients who had undergone AFib ablation, a procedure that uses heat or cold to destroy heart tissue causing irregular rhythms. Two groups were studied: one using Apple Watch for daily ECG monitoring, and another receiving standard care with clinic visits at 3, 6, and 12 months.
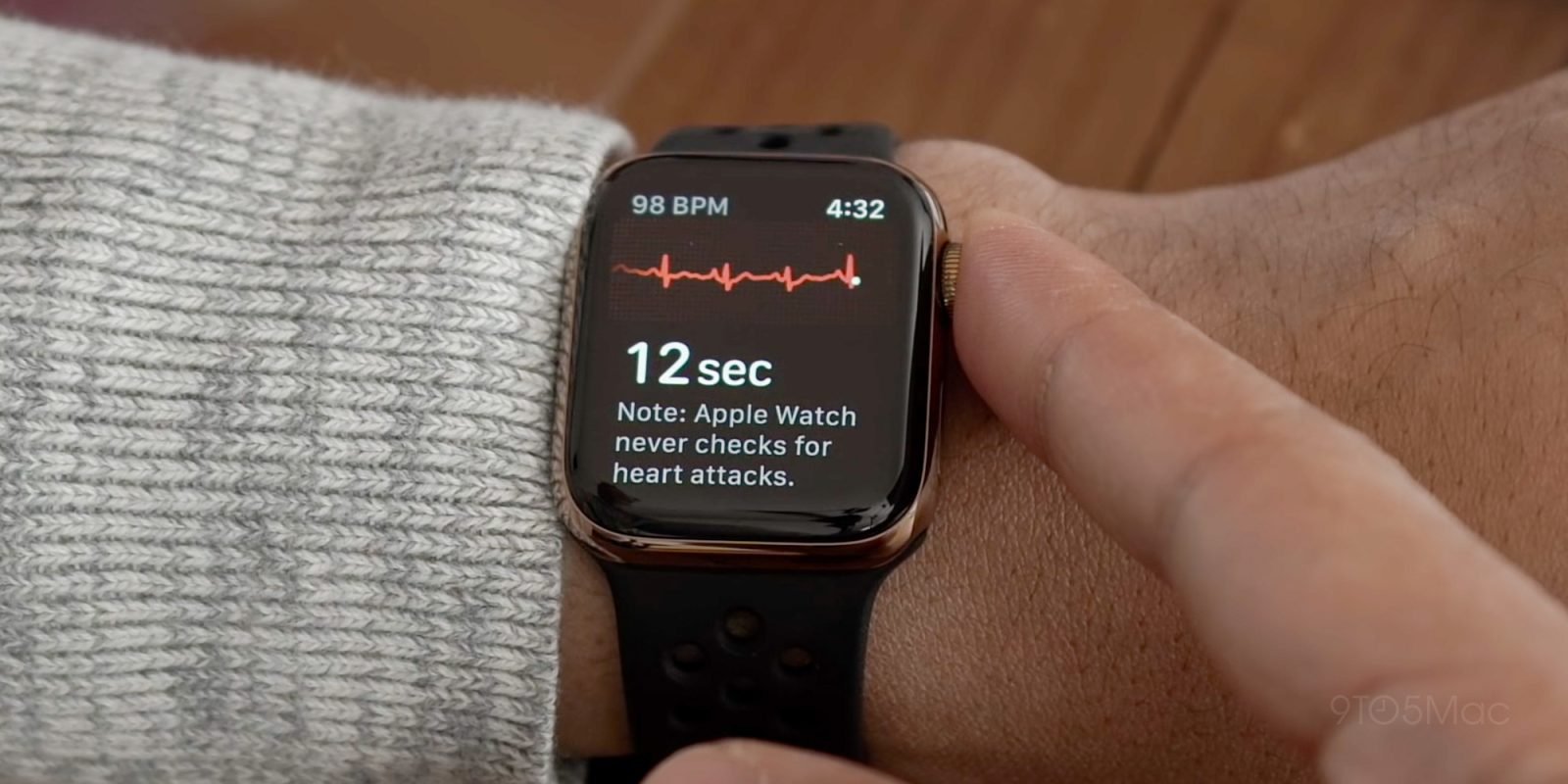
In the Apple Watch group, patients were instructed to record an ECG daily using their device, with additional recordings when they felt symptoms or received irregular rhythm notifications. A clinical team remotely reviewed all ECGs. The results were striking: after the initial 90-day "blanking period," the Apple Watch group detected AFib recurrences significantly earlier - with a median time to first confirmed recurrence of 116 days compared to 132 days in the standard care group.
The detection rate was also substantially higher. By the end of follow-up, 52.9% of patients in the Apple Watch arm had recurrences detected versus only 34.9% in the control group. This difference was primarily driven by the watch's ability to catch more paroxysmal (intermittent) episodes - the type that are notoriously difficult to detect with occasional clinic ECGs or short Holter monitoring periods.
Perhaps most notably, despite identifying more abnormalities, the Apple Watch group experienced fewer unplanned hospitalizations. This suggests that earlier detection and intervention may prevent complications that would otherwise require emergency care. The rate of repeat ablation procedures remained similar between both groups.
This study represents a significant validation of consumer health technology in clinical settings. Rather than changing the ablation procedure itself, the Apple Watch changed what patients and doctors caught afterward through continuous, patient-led monitoring integrated into daily life.
The findings align with Apple's broader health strategy, which has increasingly focused on cardiac monitoring capabilities. Since introducing ECG functionality in 2018, Apple has positioned the Apple Watch as both a preventive health tool and a monitoring device for existing conditions.
For healthcare providers, this research suggests that incorporating Apple Watch into post-ablation care protocols could improve patient outcomes while potentially reducing healthcare costs through fewer hospitalizations. For patients, it offers a less invasive alternative to traditional monitoring that fits more naturally into their daily routines.
The study's methodology - combining patient-led ECGs with remote clinical review - provides a model for how consumer health devices can be integrated into formal medical care. It demonstrates that when properly structured, these technologies can enhance rather than replace traditional medical oversight.
As wearable health technology continues to advance, studies like this will likely influence how medical institutions approach post-procedure monitoring, potentially leading to more widespread adoption of patient-led monitoring programs for various cardiac conditions.
Comments
Please log in or register to join the discussion