MIT researchers develop MagMix, a magnetically actuated mixing device that prevents cell settling in bioinks during 3D bioprinting, enabling more consistent and scalable tissue manufacturing.
3D bioprinting has emerged as a transformative technology in bioengineering, enabling researchers to create living tissues by depositing layers of bioinks—soft hydrogels mixed with living cells—to build complex tissue structures. However, a fundamental challenge has limited the scalability and consistency of this approach: gravity causes cells to sink within the bioink during printing, leading to clogged nozzles, uneven cell distribution, and inconsistent tissue quality. Researchers at MIT have now developed an innovative solution called MagMix that addresses this critical limitation, potentially accelerating the development of engineered tissues for medical applications.
The Gravity Problem in Bioprinting
The challenge stems from basic physics. Cells are denser than the hydrogel matrix surrounding them, causing them to naturally settle to the bottom of the printer syringe over time. This sedimentation becomes increasingly problematic during long print sessions required for larger tissue constructs. "This cell settling, which becomes worse during the long print sessions required to print large tissues, leads to clogged nozzles, uneven cell distribution, and inconsistencies between printed tissues," explains Ritu Raman, the Eugene Bell Career Development Professor of Tissue Engineering and assistant professor of mechanical engineering at MIT.
Traditional approaches to this problem have proven inadequate. Manual stirring before loading bioinks into the printer provides only temporary uniformity, while passive mixing systems cannot maintain consistent distribution once printing begins. These limitations have constrained the field's ability to produce reliable, high-quality tissues at scale.
How MagMix Works
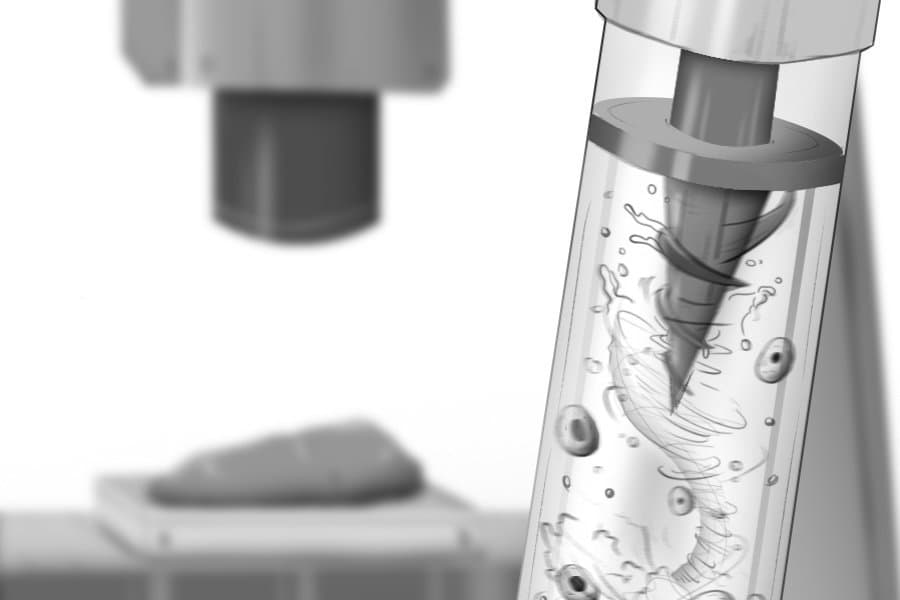
The MagMix device represents a elegant engineering solution to this persistent challenge. The system consists of two main components: a small magnetic propeller that fits inside the standard syringes used by bioprinters, and an external permanent magnet attached to a motor that moves up and down near the syringe. This external magnet controls the movement of the internal propeller, creating gentle, tunable-speed mixing in real-time without requiring any changes to the bioink formulation or interfering with the printer's normal operation.
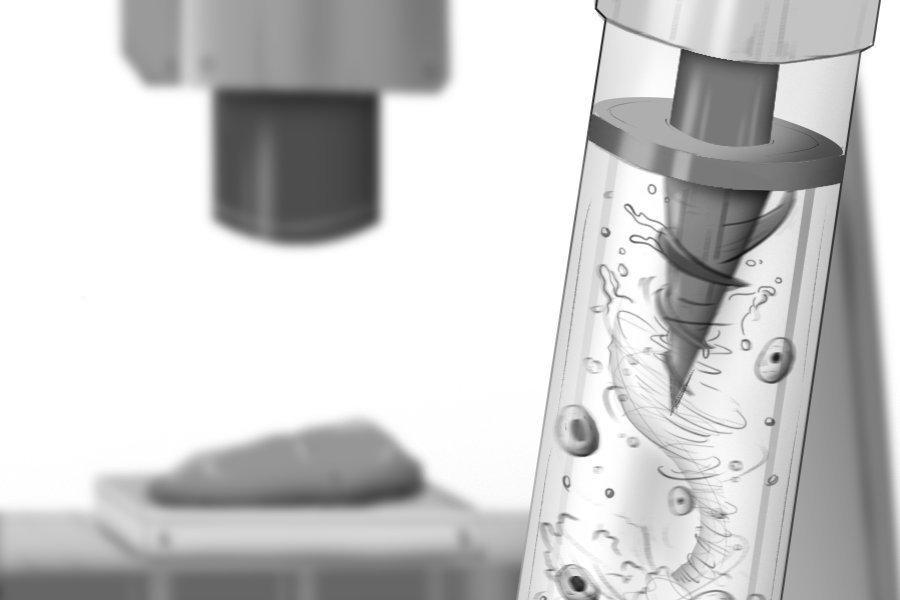
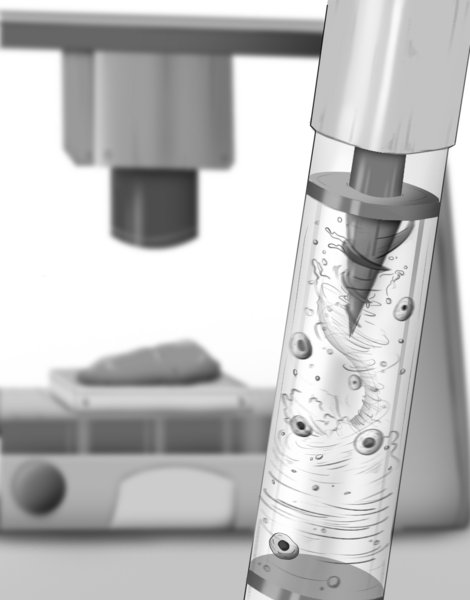
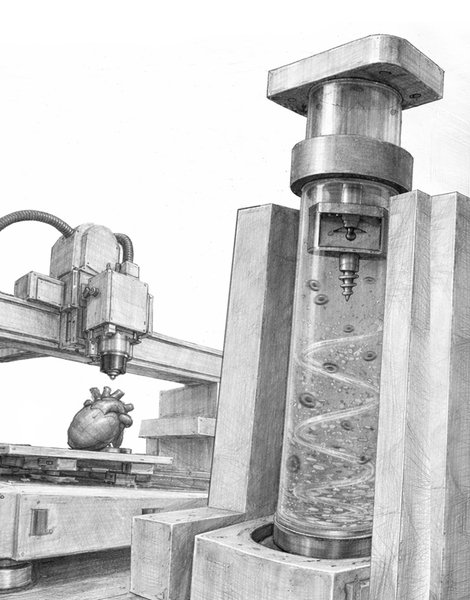
The magnetic actuation approach offers several advantages. First, it's non-invasive—the mixing occurs entirely within the syringe without requiring modifications to the printer itself. Second, the mixing speed can be precisely controlled to balance effective homogenization with minimal stress on the cells. Third, the system is compact and low-cost, making it accessible to laboratories and industries working in tissue engineering.
Validation and Performance
To optimize the device, the research team used computer simulations to design the ideal mixing propeller geometry and speed, then validated these designs through experimental testing. The results were compelling: across multiple bioink types, MagMix prevented cell settling for more than 45 minutes of continuous printing. This represents a significant improvement over existing methods and enables the fabrication of larger, more complex tissue constructs.
"Across multiple bioink types, MagMix prevented cell settling for more than 45 minutes of continuous printing, reducing clogging and preserving high cell viability," says Raman. The team also demonstrated that mixing speeds could be adjusted to accommodate different bioink viscosities and cell types while minimizing cellular stress.
As a proof-of-concept, the researchers successfully used MagMix to 3D print cells that could mature into muscle tissues over several days. This demonstration showed that the device not only maintains cell distribution during printing but also supports the subsequent development and maturation of printed tissues.
Implications for Medical Research and Treatment
The potential applications of this technology extend far beyond simply improving print quality. More consistent and reliable tissue manufacturing could accelerate progress in several critical areas of medical research and treatment.
First, engineered tissues with more uniform cell distribution can serve as better models for studying human diseases. These models could provide researchers with more accurate representations of how diseases develop and progress in human tissues, potentially leading to new therapeutic insights.
Second, the technology could enhance drug screening efforts. "If we can print tissues that more closely mimic those in our bodies, we can use them as models to understand more about human diseases, or to test the safety and efficacy of new therapeutic drugs," adds Raman. Such models could help reduce reliance on animal testing, aligning with recent initiatives from the U.S. Food and Drug Administration to develop faster, less expensive, and more informative approaches to evaluating new treatments.
Third, and perhaps most significantly, the technology moves the field closer to practical regenerative medicine applications. "Eventually, we are working towards regenerative medicine applications such as replacing diseased or injured tissues in our bodies with 3D printed tissues that can help restore healthy function," says Raman.
Supporting Infrastructure and Translation
The development of MagMix was supported by the Safety, Health, and Environmental Discovery Lab (SHED) at MIT, which provides infrastructure and interdisciplinary expertise to help translate biofabrication innovations from laboratory demonstrations to scalable, reproducible applications.
"At the SHED, we focus on accelerating the translation of innovative methods into practical tools that researchers can reliably adopt," says Tolga Durak, the SHED's founding director. "MagMix is a strong example of how the right combination of technical infrastructure and interdisciplinary support can move biofabrication technologies toward scalable, real-world impact."
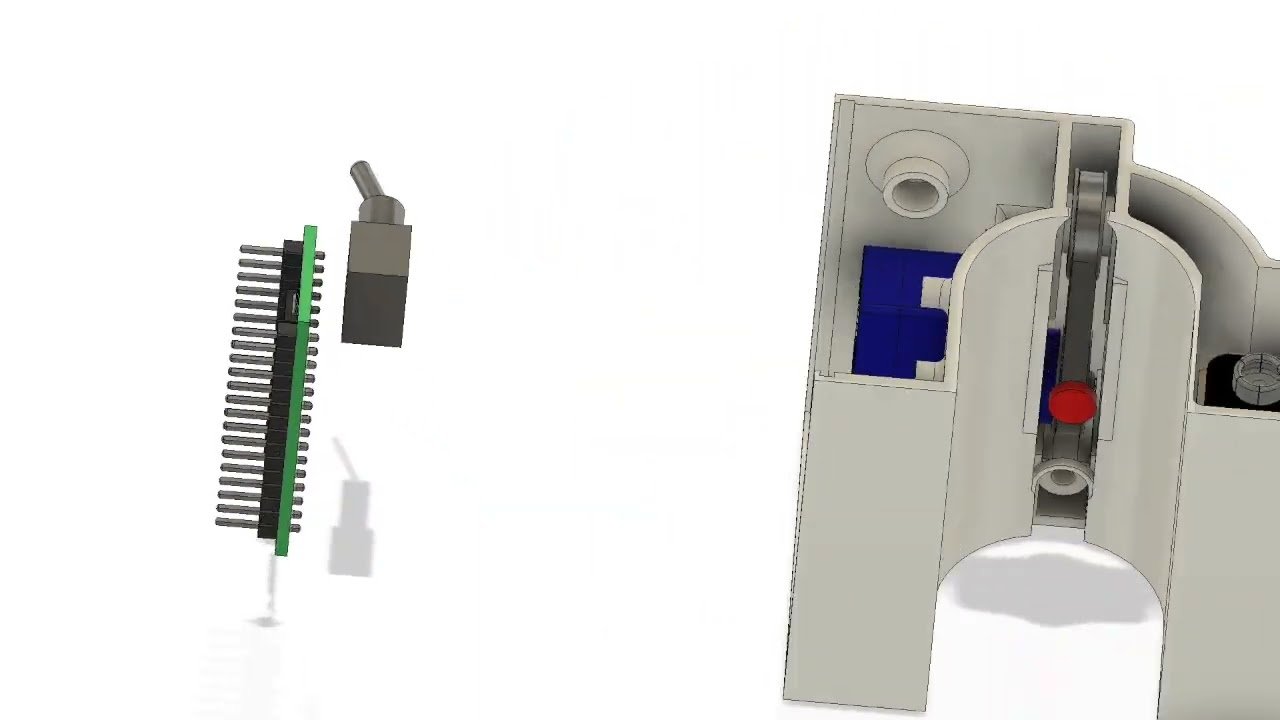
The SHED's involvement reflects a broader vision of strengthening technology pathways that enhance reproducibility and accessibility across engineering and the life sciences. By providing equitable access to advanced equipment and fostering cross-disciplinary collaboration, the lab aims to build sustainable capacity for innovation in the field.
"As the field advances toward larger-scale and more standardized systems, integrated labs like SHED are essential for building sustainable capacity," Durak adds. "Our goal is not only to enable discovery, but to ensure that new technologies can be reliably adopted and sustained over time."
Beyond Medical Applications
The researchers also see potential applications beyond traditional medical uses. One intriguing possibility is the development of "biohybrid" robots powered by printed muscle tissues. These biohybrid systems could offer advantages in safety and efficiency compared to conventional robotic actuators, potentially opening new frontiers in robotics and automation.
The Path Forward
The MagMix technology represents a significant advance in addressing one of the most persistent challenges in 3D bioprinting. By providing a simple, effective solution to cell settling, the device enables more consistent tissue manufacturing, which could accelerate progress across multiple domains of medical research and treatment.
The compact, low-cost nature of the system makes it particularly valuable, as it can be easily integrated into existing bioprinting workflows without requiring expensive equipment upgrades or extensive retraining. This accessibility could help democratize advanced tissue engineering capabilities, allowing more laboratories to pursue cutting-edge research in this field.
The research team's paper, "Advancing Bioink Homogeneity in Extrusion 3D Bioprinting with Active In Situ Magnetic Mixing," is available in the journal Device, marking an important milestone in the ongoing evolution of bioprinting technology. As the field continues to mature, innovations like MagMix will be crucial in bridging the gap between laboratory demonstrations and practical, scalable applications that can benefit human health.
For researchers and clinicians working in tissue engineering, regenerative medicine, and related fields, MagMix offers a promising tool for overcoming a fundamental technical barrier. By enabling more reliable and consistent tissue manufacturing, this technology could help accelerate the development of new treatments, improve our understanding of human disease, and ultimately contribute to better health outcomes for patients worldwide.
Comments
Please log in or register to join the discussion