Detailed radiation measurements of uranium ore samples from Utah mines using Radiacode 102 and Ludlum 44-9 detectors reveal significant activity differences across mineral types, with context on safety thresholds and regulatory considerations.
When evaluating radioactive minerals, quantitative measurements provide crucial insights into both their scientific value and practical handling requirements. Using handheld detectors favored by geology enthusiasts, we benchmarked several uranium-bearing samples from Utah's historic mining districts, comparing readings across devices while contextualizing real-world implications.
Carnonite Sample: McCormic Mine
 The carnonite specimen from McCormic Mine near Mi Vida registered 180 CPS (counts per second) and 4 μSv/h on the Radiacode 102 gamma spectrometer. The Ludlum 44-9 pancake detector—optimized for alpha/beta particles—recorded 20,000 CPM (counts per minute). This yellow-mineralized sample was plastic-coated to contain radioactive dust, a standard safety practice. Carnonite's chemical composition (K₂(UO₂)₂(VO₄)₂·3H₂O) typically yields moderate radiation due to uranium decay chains.
The carnonite specimen from McCormic Mine near Mi Vida registered 180 CPS (counts per second) and 4 μSv/h on the Radiacode 102 gamma spectrometer. The Ludlum 44-9 pancake detector—optimized for alpha/beta particles—recorded 20,000 CPM (counts per minute). This yellow-mineralized sample was plastic-coated to contain radioactive dust, a standard safety practice. Carnonite's chemical composition (K₂(UO₂)₂(VO₄)₂·3H₂O) typically yields moderate radiation due to uranium decay chains.
Uraninite-Flecked Sandstone: Mi Vida
 A sandstone specimen with uraninite (UO₂) inclusions from the same region showed dramatically higher activity: 1,700 CPS and 40 μSv/h on the Radiacode 102, with the Ludlum 44-9 hitting 70,000 CPM. At 15 cm distance, radiation dropped to 50 CPS (1 μSv/h), detectable from one meter away by prospecting equipment. Using gamma dose constants, this suggests approximately 10-20 grams of uranium content—though mineralization heterogeneity makes this estimate approximate.
A sandstone specimen with uraninite (UO₂) inclusions from the same region showed dramatically higher activity: 1,700 CPS and 40 μSv/h on the Radiacode 102, with the Ludlum 44-9 hitting 70,000 CPM. At 15 cm distance, radiation dropped to 50 CPS (1 μSv/h), detectable from one meter away by prospecting equipment. Using gamma dose constants, this suggests approximately 10-20 grams of uranium content—though mineralization heterogeneity makes this estimate approximate.
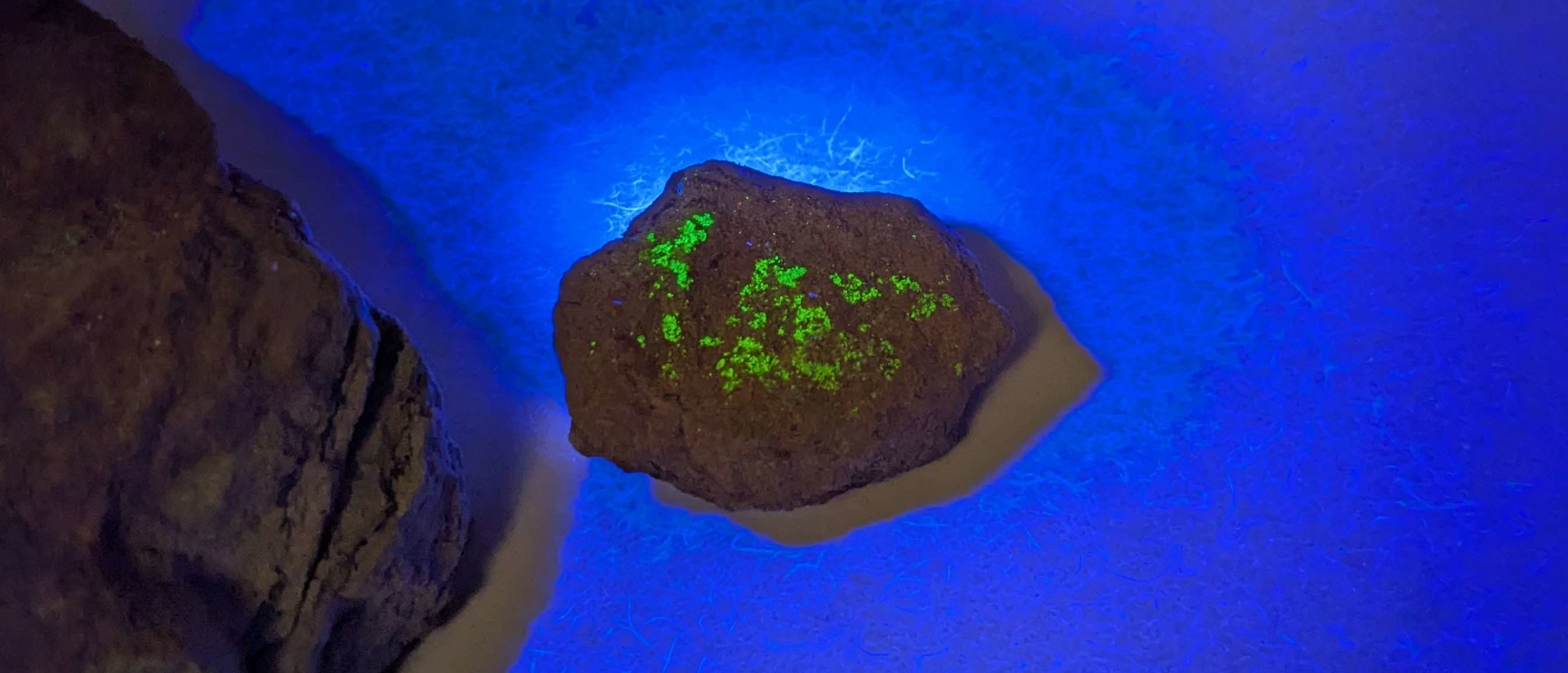
Fluorescing U(IV) Mineral: Yellow Cat Claims
 An unidentified U(IV) mineral from Parco claims in Yellow Cat measured lower activity (2 CPS on Radiacode 102, 350 CPM on Ludlum 44-9) but exhibited intense green fluorescence under 365 nm UV light—a characteristic of many uranium minerals. Contrary to Hollywood depictions, spent nuclear fuel doesn't glow; this phenomenon arises from uranium's interaction with crystal lattices.
An unidentified U(IV) mineral from Parco claims in Yellow Cat measured lower activity (2 CPS on Radiacode 102, 350 CPM on Ludlum 44-9) but exhibited intense green fluorescence under 365 nm UV light—a characteristic of many uranium minerals. Contrary to Hollywood depictions, spent nuclear fuel doesn't glow; this phenomenon arises from uranium's interaction with crystal lattices.

Radiation Context and Safety
All specimens emit less ambient radiation than common household sources (e.g., granite countertops average 0.3 μSv/h). While external exposure poses minimal risk, uranium's chemical toxicity makes ingestion hazardous. Under U.S. regulations (10 CFR §40.13(b)), naturally occurring radioactive materials (NORM) like these often fall outside strict licensing requirements, but local laws vary.
Non-Radioactive Counterparts
Jasper/agate samples from Yellow Cat and petrified wood near McCormic Mine showed background radiation levels (<0.1 μSv/h), confirming their suitability for decorative use without radiological concerns.
Detector Performance Notes
- Radiacode 102: Gamma-focused spectrometer ideal for dose rate (μSv/h) and isotope identification via CPS.
- Ludlum 44-9: Pancake Geiger-Müller tube excels at detecting alpha/beta particles (CPM), crucial for surface contamination checks.
For collectors, pairing both devices provides comprehensive hazard assessment. Always prioritize containment (e.g., acrylic cases) and avoid inhalation/ingestion. These benchmarks underscore uranium ore's fascinating variability—a testament to nature's unregulated laboratory.
Disclaimer: Radiation measurements reflect specific samples; actual values depend on mineral concentration and geometry. Consult local authorities before collecting radioactive materials.

Comments
Please log in or register to join the discussion