MIT and Scripps researchers develop DNA-based virus-like particles that dramatically outperform protein scaffolds in generating rare B cells needed for broadly neutralizing HIV antibodies.
Researchers at MIT and the Scripps Research Institute have developed a novel vaccine platform using DNA origami to create virus-like particles (VLPs) that generate significantly more rare B cells capable of producing broadly neutralizing antibodies against HIV and potentially other challenging pathogens.
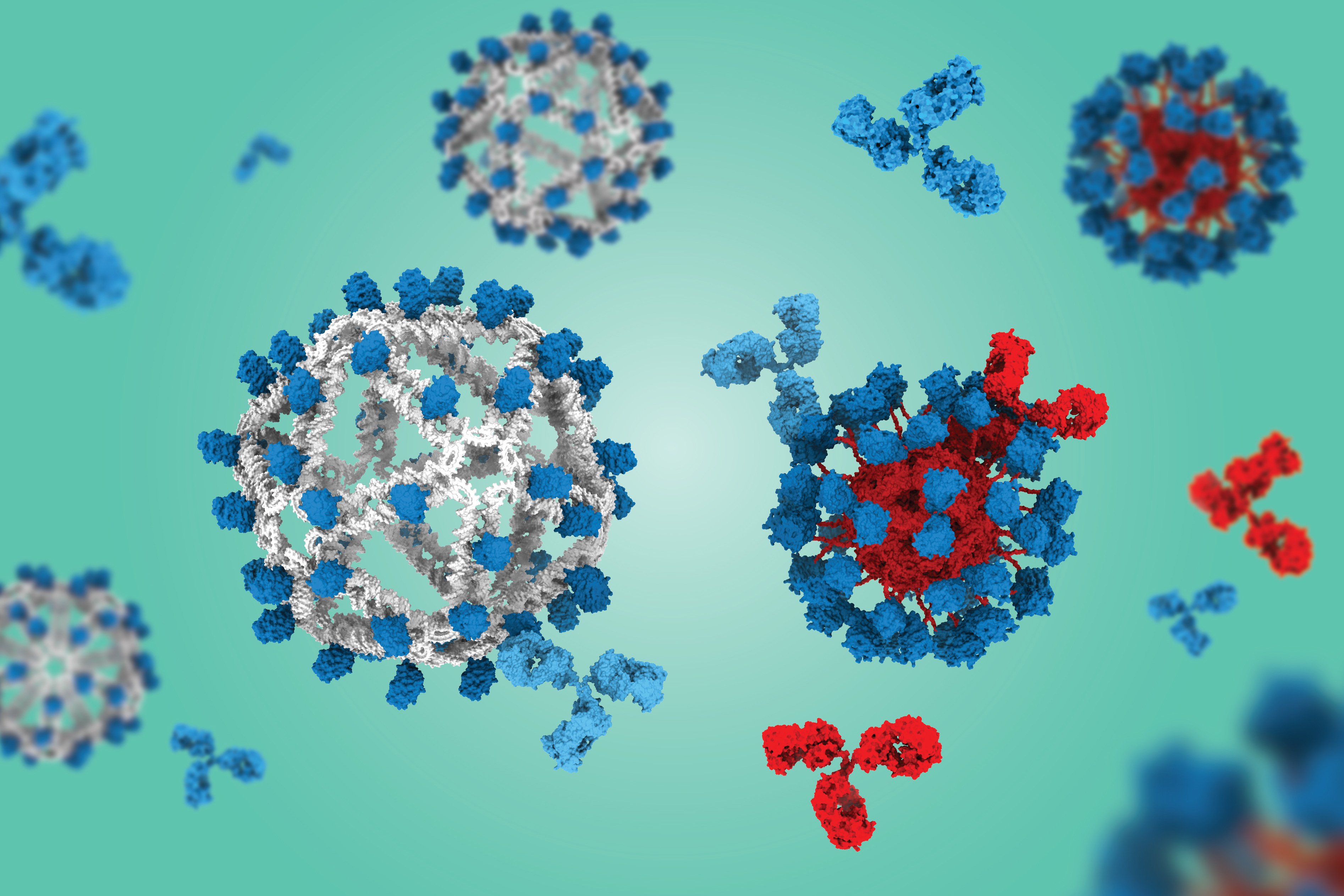
The breakthrough addresses a longstanding challenge in HIV vaccine development: the need to expand extremely rare precursor B cells that can evolve to produce antibodies capable of neutralizing multiple HIV strains. These broadly neutralizing antibodies are particularly valuable because HIV mutates rapidly to evade immune responses.
DNA vs. Protein Scaffolds
The key innovation involves using DNA instead of protein as the scaffold for fabricating VLPs that display engineered HIV antigens. In preclinical studies using humanized mouse models, the DNA-based VLPs generated eight times more "on-target" B cells compared to a protein-based VLP that has already shown success in human clinical trials.
"We were all surprised that this already outstanding VLP from Scripps was significantly outperformed by the DNA-based VLP," says Mark Bathe, an MIT professor of biological engineering. "These early preclinical results suggest a potential breakthrough as an entirely new, first-in-class VLP that could transform the way we think about active immunotherapies, and vaccine design, across a variety of indications."
The HIV Target
The vaccine displays an engineered HIV immunogen called eOD-GT8, developed at Scripps. This antigen is designed to prime B cells that can eventually evolve into VRC01-like antibodies - a class of broadly neutralizing antibodies discovered in HIV patients who never developed AIDS.
Generating these antibodies requires a three-stage vaccination process, with each stage using different antigens to guide B cell evolution. The eOD-GT8 antigen serves as the priming stage, initiating the process by expanding the rare precursor B cells.
The "Silent" Advantage
A critical advantage of the DNA scaffold is that it doesn't induce an immune response against the particle itself. Protein-based VLPs often generate "off-target" antibodies that bind to the scaffold rather than the desired antigen, potentially interfering with the vaccine's effectiveness.
"The DNA-VLP allowed us for the first time to assess whether B cells targeting the VLP itself limit the development of 'on target' B cell responses - a longstanding question in vaccine immunology," explains Darrell Irvine, a professor at Scripps Research Institute.
Technical Details
The DNA origami approach provides precise control over the VLP structure. The researchers found that a smaller diameter version with 60 copies of the antigen (compared to 30 in earlier versions) dramatically outperformed the clinical protein VLP construct. This improvement resulted from better retention in B cell follicles in lymph nodes and enhanced collaboration with helper T cells.
Beyond HIV
The researchers suggest this DNA-VLP platform could be applied to other challenging vaccine targets, including influenza and potentially chemical warfare agents. It might also serve as an active immunotherapy for generating antibodies against amyloid beta or tau proteins in Alzheimer's disease, or against addictive substances like opioids and nicotine.
"While nanoparticle formulations have been great at boosting antibody responses to various antigens, there is always this nagging question of whether competition from B cells specific for the particle's own structural antigens won't get in the way of antibody responses to targeted epitopes," notes Gabriel Victora, a professor at Rockefeller University who was not involved in the study.
The research was funded by multiple institutions including the National Institutes of Health, the Gates Foundation Collaboration for AIDS Vaccine Discovery, and the Howard Hughes Medical Institute. The findings appear in the journal Science, with Anna Romanov PhD '25 as lead author.
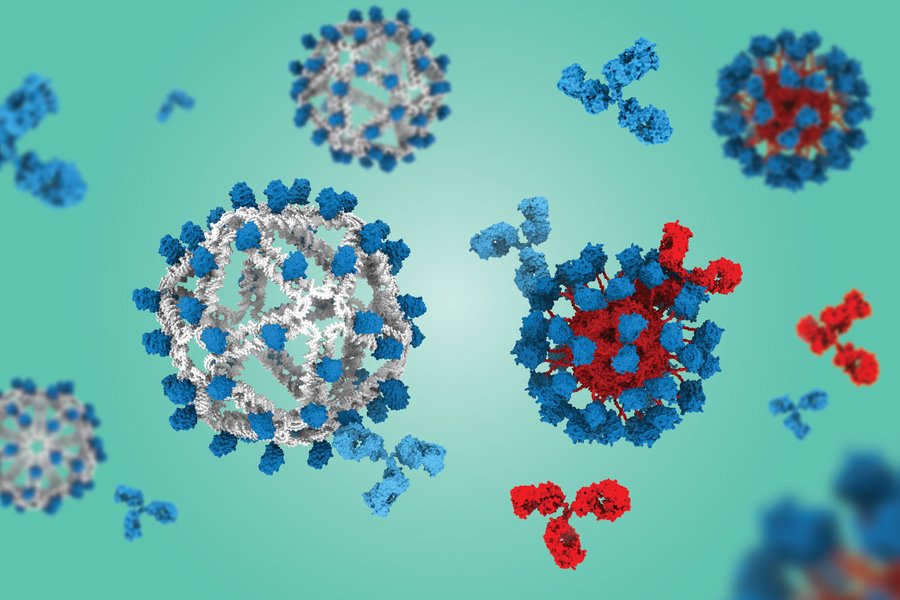
This DNA-based approach represents a fundamental shift in vaccine design, offering a "silent" scaffold that could enable more focused immune responses across a wide range of therapeutic applications.
Comments
Please log in or register to join the discussion