A groundbreaking study demonstrates that a single dose of AAV-delivered broadly neutralizing antibodies (bNAbs) administered at birth provides durable HIV protection for over three years in infant primates. The research leverages neonatal immune tolerance to overcome anti-drug antibody challenges that typically limit efficacy in adults, with significant implications for preventing perinatal HIV transmission.
Newborn primates given a single injection of adeno-associated virus (AAV) vectors encoding HIV-1 broadly neutralizing antibodies (bNAbs) developed sustained immunity that protected them against simian-HIV infection for years, according to a landmark study published in Nature. This approach capitalizes on the unique immunological properties of early infancy to achieve what could become a ‘one-shot’ solution for preventing pediatric HIV infections in high-burden regions.
The Vertical Transmission Challenge
Despite advances in antiretroviral therapy (ART), over 100,000 children still acquire HIV-1 annually, primarily through vertical transmission in sub-Saharan Africa. Complex socioeconomic barriers—including limited ART access postpartum and poor adherence among young mothers—continue to fuel infections. Current antibody therapies require repeated infusions, making them impractical in resource-limited settings.
# Simplified mechanism of AAV-bNAb delivery
vector = AAV8(serotype=8)
vector.insert_transgene(rh-3BNC117_IgG1_LS) # Bicistronic bNAb construct
delivery = intramuscular_injection(neonate, vector)
# Results in sustained myocyte production of HIV-neutralizing antibodies
Key Findings from Primate Studies
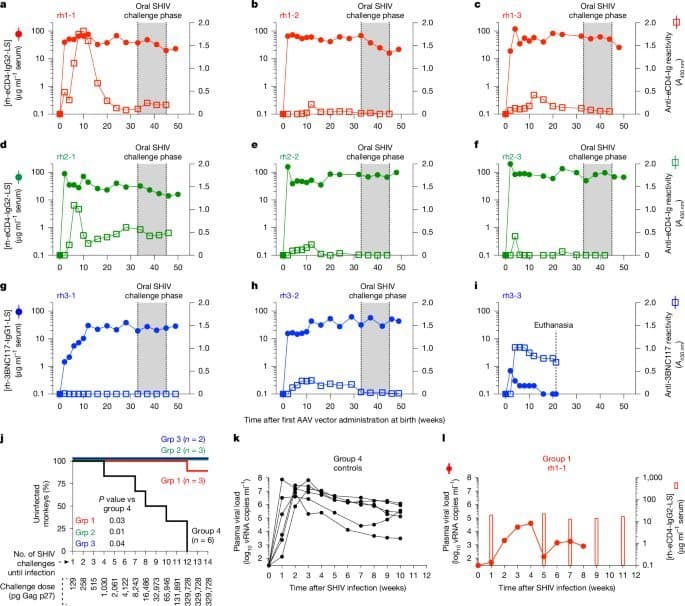
- Durable Protection from Single Dose: Infant rhesus macaques receiving AAV8-rh-3BNC117-IgG1-LS vectors intramuscularly at birth maintained therapeutic bNAb levels (>10 µg/mL) for over three years without redosing.
- Age as Critical Determinant: Treatment success inversely correlated with age at administration. Neonates (0-4 weeks) showed significantly higher persistent bNAb expression (5/5 success) versus juveniles (2/6) due to reduced anti-drug antibody (ADA) development:
"The neonatal immune system’s predisposition toward tolerance—rather than immunity—allows sustained acceptance of vectored antibodies," noted lead researcher Mauricio Martins.
- In Utero Tolerance Induction: Prenatal exposure to recombinant bNAbs eliminated ADA responses in older infants (8-12 weeks), enabling successful postnatal AAV-bNAb therapy in 8/8 subjects versus 2/5 controls (p=0.035).
- Efficacy Against Real-World Challenges: Treated infants resisted:
- Oral SHIV challenges mimicking breastfeeding transmission (87.5% protection)
- Rectal SHIV challenges in adolescence mimicking sexual transmission (83.3% protection)
Technical and Implementation Implications
- Thermostability: AAV vectors’ resilience facilitates deployment in regions with cold-chain limitations.
- Capsid Serotype Selection: Critical due to varying pre-existing immunity; muscle-directed AAV8 tolerates higher NAb titers than hepatic delivery.
- Scalability Hurdles: Current AAV manufacturing costs remain prohibitive, though initiatives to expand production in LMICs are underway.
The Path Forward
This research demonstrates that immunological windows in early development can be harnessed for durable infectious disease prevention. While challenges in vector affordability and capsid engineering persist, the approach holds promise not just for HIV but for other pediatric pathogens like malaria. Clinical trials evaluating neonatal AAV-bNAb delivery in humans are the essential next step.
Source: Ardeshir et al. Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life. Nature (2025). DOI: 10.1038/s41586-025-09330-2
Comments
Please log in or register to join the discussion