A newly characterized protein called intelectin-2 strengthens the intestinal mucus barrier while directly neutralizing antibiotic-resistant bacteria, offering a novel therapeutic approach for gastrointestinal disorders and infections.
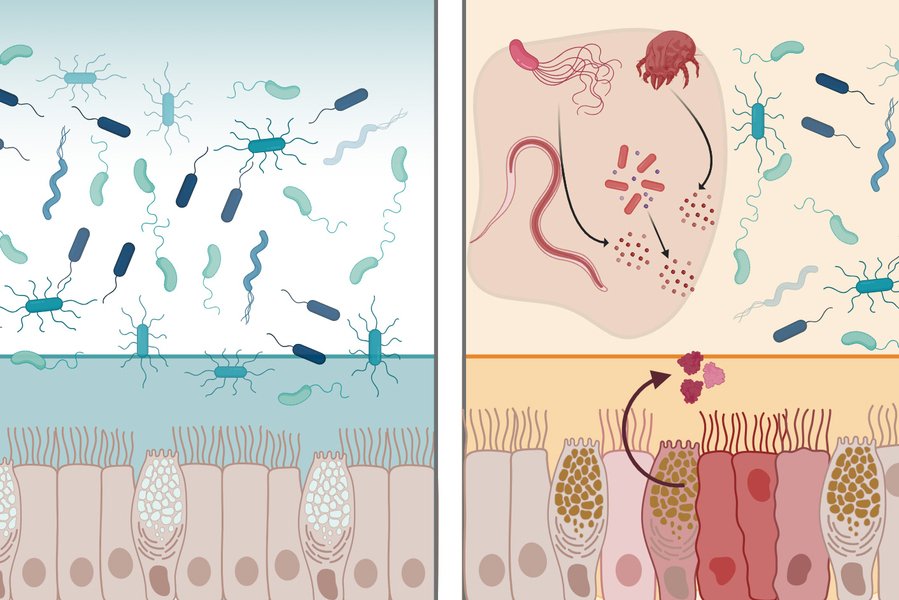
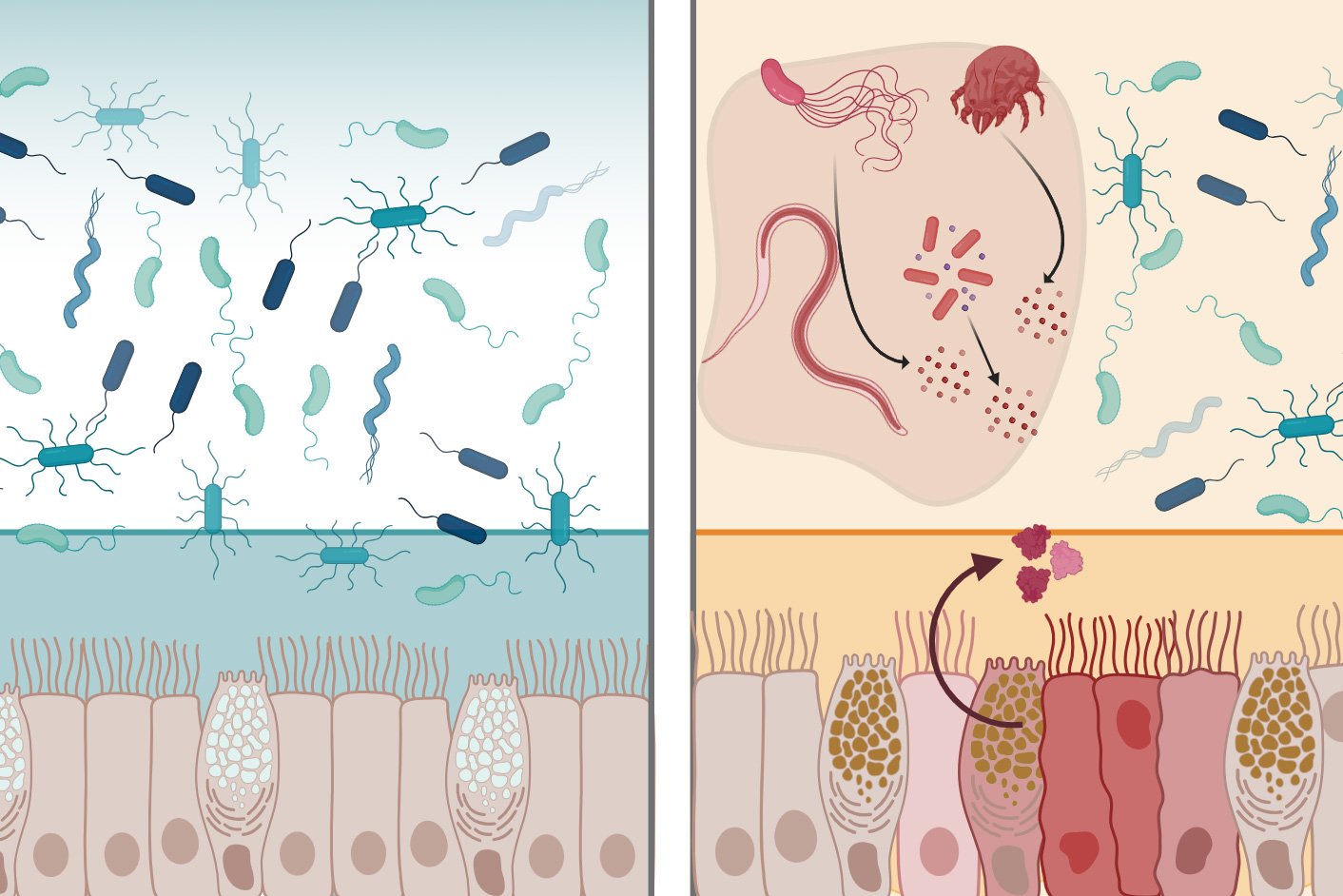
The human digestive tract maintains a delicate balance, hosting trillions of microbes while preventing dangerous pathogens from causing infection. Central to this defense is the mucus layer lining the intestines—a viscous barrier that physically blocks microbes and contains antimicrobial molecules. MIT researchers have now characterized a protein called intelectin-2 (mltln2) that operates through two distinct mechanisms to protect the GI tract, potentially opening new therapeutic avenues for conditions like inflammatory bowel disease and antibiotic-resistant infections.
Published today in Nature Communications, the study reveals that intelectin-2 both reinforces the mucus barrier and directly attacks bacteria that breach it. "What's remarkable is that intelectin-2 operates in two complementary ways. It helps stabilize the mucus layer, and if that barrier is compromised, it can directly neutralize or restrain bacteria that begin to escape," explains Laura Kiessling, the Novartis Professor of Chemistry at MIT and the study's senior author.
A Lectin with Dual Antimicrobial Mechanisms
Intelectin-2 belongs to a family of carbohydrate-binding proteins called lectins, which the human genome encodes in over 200 varieties. These proteins recognize microbes by binding to specific sugar molecules on cell surfaces. Kiessling's lab has studied lectin-carbohydrate interactions for years, recently focusing on intelectins—two closely related proteins in humans (intelectin-1 and intelectin-2).
The researchers discovered that both human and mouse intelectin-2 bind to galactose, a sugar molecule found in two critical contexts: within mucins that compose the mucus layer, and on the surfaces of many pathogenic bacteria. This dual recognition enables the protein's two-pronged defense strategy.
First, by binding galactose-containing mucins, intelectin-2 crosslinks mucus molecules, creating a more robust physical barrier. Second, when bacteria attempt to penetrate this barrier, intelectin-2 binds to galactose on their membranes, effectively trapping them and disrupting their cell walls. The researchers observed that trapped microbes eventually disintegrate, suggesting the protein kills them by compromising membrane integrity.
This broad-spectrum activity affects diverse bacterial species, including pathogens resistant to conventional antibiotics. The mechanism differs from traditional antibiotics, which typically target specific bacterial processes. Instead, intelectin-2 exploits a fundamental vulnerability—surface carbohydrates—making it harder for bacteria to develop resistance.
Therapeutic Implications for GI Disorders
The discovery has particular relevance for inflammatory bowel disease (IBD), where intelectin-2 levels fluctuate abnormally. Patients with IBD often show degraded mucus barriers, allowing bacteria to contact intestinal epithelium directly and trigger inflammation. The researchers hypothesize that low intelectin-2 levels could contribute to this barrier breakdown.
Conversely, excessively high intelectin-2 might eliminate beneficial gut bacteria essential for digestion and immune function. This delicate balance suggests that therapeutic applications would require precise modulation rather than simple supplementation.
"Our findings show just how critical it is to stabilize the mucus barrier. Looking ahead, we can imagine exploiting lectin properties to design proteins that actively reinforce that protective layer," Kiessling notes.
The protein's ability to neutralize difficult pathogens like Staphylococcus aureus and Klebsiella pneumoniae—both notorious for antibiotic resistance—makes it particularly valuable. These bacteria cause severe hospital-acquired infections that are increasingly difficult to treat with existing drugs.

Harnessing Innate Immunity
The research builds on growing interest in leveraging the body's own defense mechanisms rather than developing entirely new compounds. "Harnessing human lectins as tools to combat antimicrobial resistance opens up a fundamentally new strategy that draws on our own innate immune defenses," Kiessling explains. "Taking advantage of proteins that the body already uses to protect itself against pathogens is compelling and a direction that we are pursuing."
This approach could involve several therapeutic strategies:
Direct administration: Intelectin-2 could be delivered as a protein therapeutic to reinforce mucus barriers in IBD patients or to treat active infections.
Lectin engineering: Modified versions could be designed to target specific pathogens while preserving beneficial microbiota.
Mucus mimetics: Synthetic mucus incorporating intelectin-2 or similar lectins could protect vulnerable patients, such as those undergoing chemotherapy or with compromised immune systems.
Research Context and Collaborators
The study involved a multidisciplinary team including Amanda Dugan, a former MIT research scientist, and Deepsing Syangtan PhD '24 as lead authors. Collaborators included Charles Bevins (UC Davis), Ramnik Xavier (Harvard Medical School and Broad Institute), and Katharina Ribbeck (MIT Biological Engineering).
Funding came from multiple NIH institutes (Glycoscience Common Fund, Allergy and Infectious Disease, General Medical Sciences) and the National Science Foundation, reflecting the work's fundamental importance to immunology and microbiology.
Moving Toward Clinical Application
While intelectin-2 shows promise, several challenges remain before clinical use. Researchers must determine optimal dosing, delivery methods, and potential side effects. The protein's broad activity could disrupt beneficial gut flora if not properly controlled. Additionally, developing stable formulations that survive the harsh GI environment will be crucial.
The researchers are also investigating intelectin-1, which has similar structure but different carbohydrate specificity. Understanding how these related lectins coordinate their activities could reveal broader principles of mucosal immunity.
For patients with IBD, chronic infections, or compromised gut barriers, intelectin-2-based therapies could offer an alternative to antibiotics that preserves microbiome health while actively reinforcing natural defenses. As antibiotic resistance escalates globally, such approaches that work with rather than against biological systems may prove essential.
The study represents a significant step toward translating basic research on lectin-carbohydrate interactions into practical therapeutic strategies, demonstrating how understanding fundamental molecular mechanisms can reveal new paths for treating complex diseases.
Paper: "Intelectin-2 is a broad-spectrum antimicrobial lectin" - Nature Communications (2026)
Related Research: MIT Department of Chemistry, Ribbeck Lab, Kiessling Lab
Comments
Please log in or register to join the discussion