Researchers are harnessing mitochondria—the cell's power generators—for revolutionary organ repair. Successful trials in infant heart surgery and animal studies show transplanted mitochondria can reverse tissue damage caused by oxygen deprivation, with potential applications in stroke, organ transplantation, and emergency medicine. This experimental therapy could transform treatment for ischemia-reperfusion injuries affecting millions worldwide.
Mitochondrial Transplants: The Emerging Biotech Frontier in Healing Damaged Organs
When James McCully injected extracted mitochondria into a dying pig heart in his Harvard lab two decades ago, he witnessed the impossible: Within minutes, the gray, failing organ regained its rosy hue and resumed beating. This serendipitous experiment ignited a new field of medical biotechnology—mitochondrial transplantation—now showing remarkable promise in treating organ damage from oxygen deprivation.
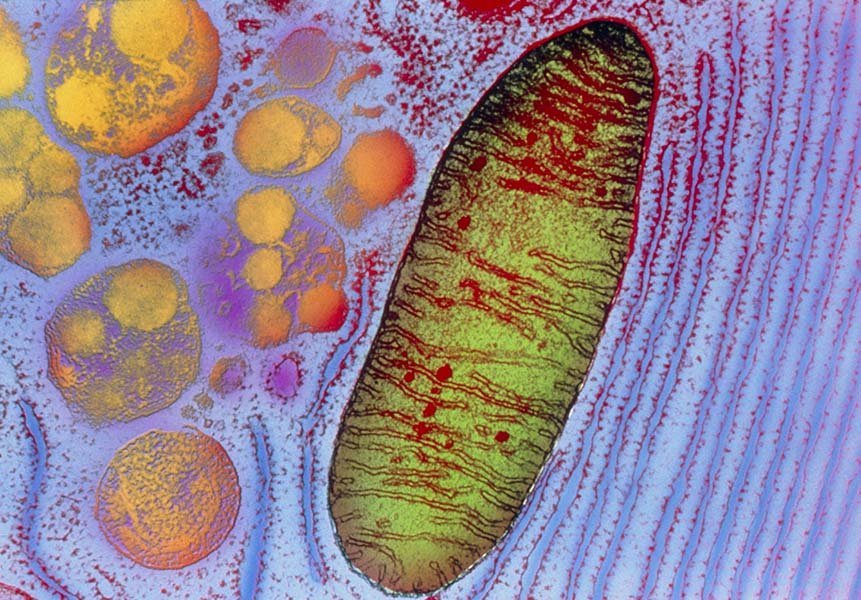
Caption: A transmission electron micrograph reveals the intricate structure of a mitochondrion—far more than just a cellular battery. These organelles regulate immune responses, cell death pathways, and stress management. (Credit: Keith R. Porter / Science Source)
Beyond Energy Production: Mitochondria as Cellular Therapists
While famously dubbed "the powerhouse of the cell," mitochondria serve as sophisticated signaling hubs that maintain biological equilibrium. When tissues are deprived of blood flow (ischemia), their mitochondria falter, triggering cell death. Restoring blood flow often worsens damage through inflammation—a phenomenon called ischemia-reperfusion injury affecting heart attack and stroke patients, organ transplant recipients, and trauma cases.
Mitochondrial transplantation leverages these organelles' dual roles:
- Bioenergetic resuscitation: Donor mitochondria boost ATP production in energy-starved cells
- Molecular reprogramming: They modulate inflammatory pathways and reduce cell death signals
From Lab Bench to Operating Room
Infant Heart Surgery Breakthrough
Cardiac surgeon Sitaram Emani partnered with McCully to address a dire problem: Babies suffering heart damage after complex surgeries. During operations, stopping blood flow can cause ischemia-reperfusion injury, leaving some infants unable to wean off life support.
Their pioneering approach:
- Harvest mitochondria from the patient's own leg muscle during surgery
- Isolate functional mitochondria via filtration
- Inject directly into damaged heart tissue
Results from their 2015-2018 trial were striking: 80% of treated infants (8/10) regained cardiac function versus 29% in historical controls. Recovery time plummeted from 9 days to just 2 days. The team has since performed 17 successful transplants.
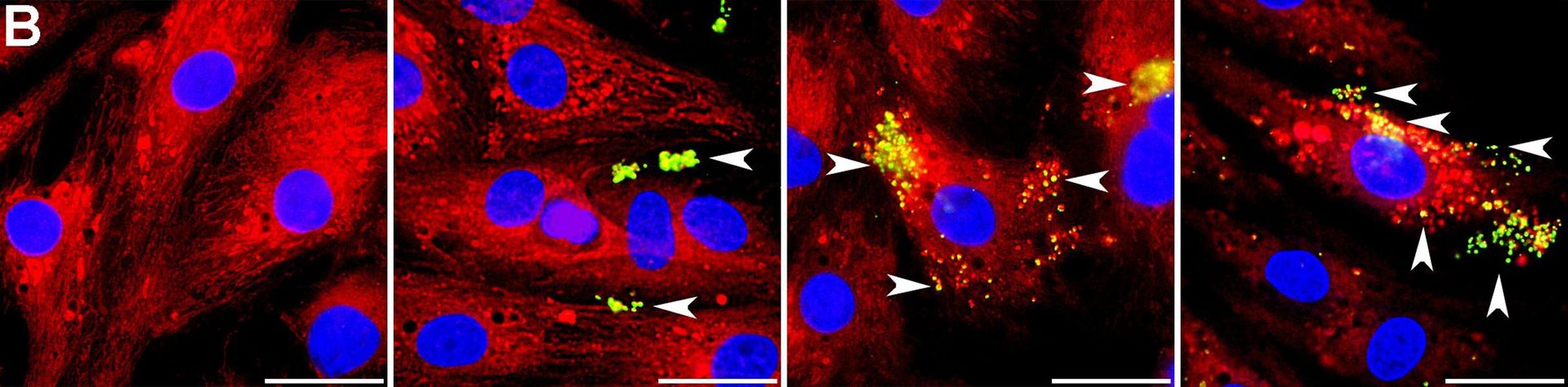
Caption: Microscopic evidence shows transplanted mitochondria (yellow-green) integrating into rat heart cells within hours—a cellular rescue mission. (Credit: A. Masuzawa et al / American Journal of Physiology–Heart and Circulatory Physiology 2013)
Expanding the Frontier
- Stroke Intervention: Neurosurgeon Melanie Walker used arterial catheters to deliver mitochondria to stroke patients' brains. Early trials confirmed safety, with efficacy studies underway. As Walker notes: "You see patients lose their ability to walk or talk. You just want to do better."
- Organ Transplantation: Wake Forest researchers reduced cell death in pig kidneys by 40% using mitochondrial infusion—potentially expanding the pool of viable donor organs.
- Cardiac Arrest: Northwell Health studies show functional mitochondria improve survival and neurological outcomes in rats post-arrest.
Technical Hurdles and Mechanistic Mysteries
Despite promising results, significant challenges remain:
Key Challenges in Mitochondrial Therapy
--------------------------------------
| Challenge | Current Status | Required Advancements |
|--------------------|------------------------------|--------------------------------|
| Mitochondrial Viability | Functional mitochondria required | Cryopreservation techniques |
| Delivery Methods | Direct injection/catheters | Targeted nanoparticle systems |
| Scaling Production | Lab-scale isolation | Automated bioreactor platforms |
| Mechanism Clarity | Signaling effects observed | Pathway mapping via omics |
Northwestern University's Navdeep Chandel questions whether donor mitochondria directly replace damaged ones or act as signaling triggers: "The big question is, what's the mechanism?" Meanwhile, Berkeley mitochondrial biologist Koning Shen highlights scaling hurdles: "Making treatments a larger reality requires solving storage and preservation."
The Road to Clinical Translation
Researchers envision a future with "mitochondria banks"—universal supplies of stored, transplant-ready organelles. As Northwell Health's Lance Becker observes: "We're so much at the beginning... but we know it's doing something mighty darn interesting." Before FDA approval, however, the field must:
- Validate mechanisms through advanced imaging and single-cell analysis
- Conduct larger human trials across organ systems
- Develop standardized mitochondrial characterization protocols
This biotechnological approach exemplifies how understanding subcellular biology can yield macroscopic medical breakthroughs. With ischemia-reperfusion injuries contributing to 45% of all hospital deaths in developed nations, mitochondrial transplantation represents more than scientific curiosity—it's a potential paradigm shift in regenerative medicine.
Source: Adapted from Knowable Magazine (July 2025), "Mighty mitochondria: Cell powerhouses harnessed for healing" by Jackie Rocheleau.

Comments
Please log in or register to join the discussion