A practical guide to building a spinthariscope using Americium-241 from a smoke detector and a ZnS(Ag) scintillator screen, demonstrating how individual alpha particle decays can be observed as visible sparks of light.
Radioactive decay is one of the few atomic processes violent enough to be directly observed by the human eye. When an atom decays, it can eject an alpha particle—a helium nucleus—with kinetic energy in the range of 4-9 MeV. While this represents only about a picojoule of energy, it's enough to produce a brief, visible flash of light when it strikes certain materials. This phenomenon is the basis of the spinthariscope, a simple instrument that makes individual radioactive decays visible as a sea of sparks.
The Components: Americium-241 and Zinc Sulfide
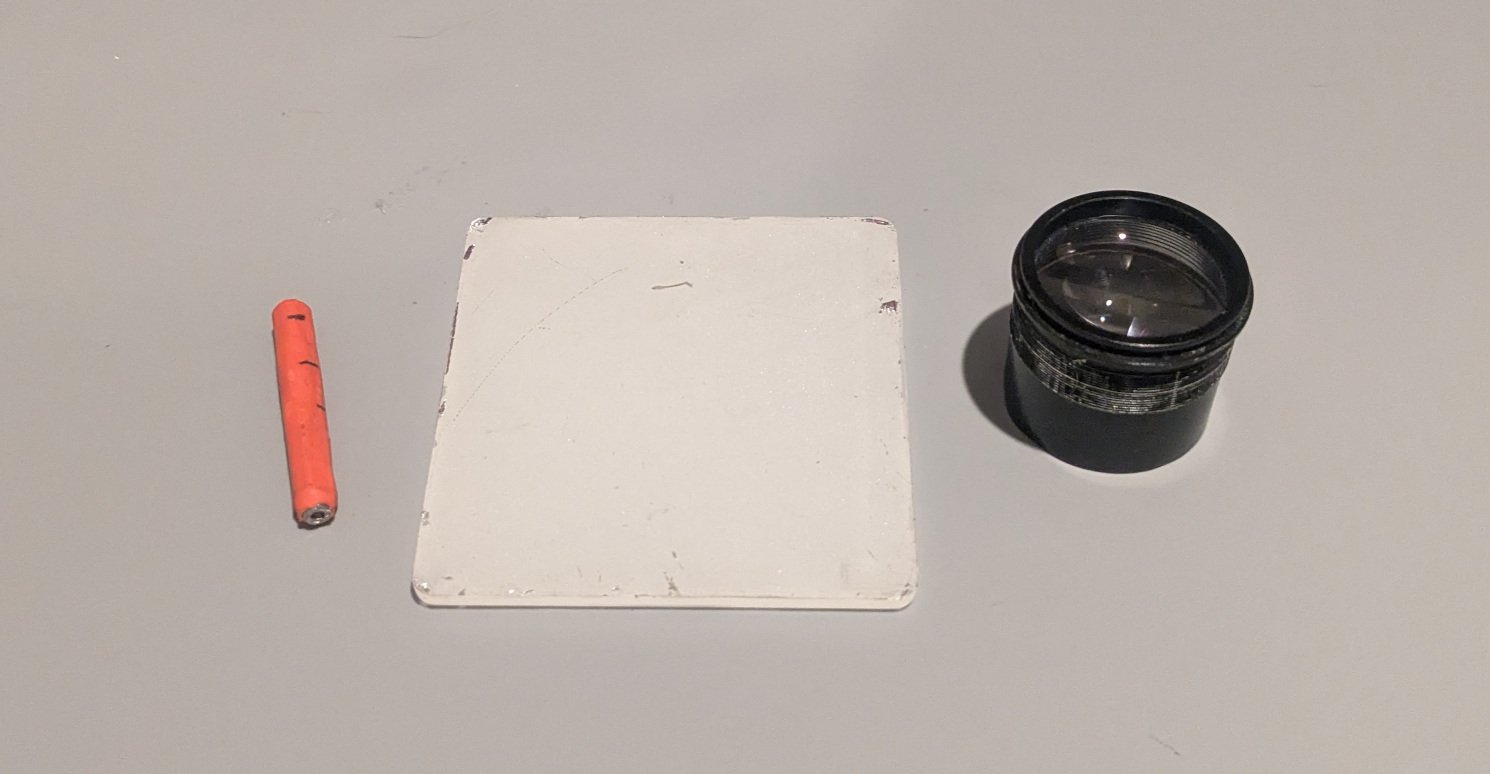
The core of any spinthariscope is a radioactive alpha source and a scintillator screen. For the source, I used a 37 kBq Americium-241 source extracted from a smoke detector. Americium-241 is a common alpha emitter with a half-life of 432 years, making it a stable and relatively safe source for experimentation. The activity is low enough that basic safety precautions—primarily avoiding ingestion or inhalation—are sufficient. Other viable sources include old radium watch hands (which emit alpha particles) or surface-mineralized pieces of uranium ore.
The scintillator is a square of plastic coated with zinc sulfide doped with silver (ZnS(Ag)). This material is chosen because it efficiently converts the kinetic energy of alpha particles into visible light through a process called scintillation. When an alpha particle strikes the ZnS(Ag) crystal lattice, it excites electrons, which then de-excite by emitting photons in the blue-green spectrum. Each alpha particle typically produces a few thousand photons, which is just enough for the human eye to detect under optimal conditions.
The Setup: Minimalist Radiation Detection
The experimental setup is elegantly simple: no power source, no electronics, just physics. The Americium source is placed a few millimeters away from the ZnS(Ag) screen. The distance is critical—too far and the alpha particles won't reach the screen (they have a range of only a few centimeters in air), too close and the geometry becomes problematic.
A magnifying glass is essential. It serves two purposes: it magnifies the tiny flashes of light, and more importantly, it concentrates more light into the eye by reducing the viewing angle. Without magnification, the individual flashes are too faint to resolve against the background darkness.
The Observation: A Roiling Sea of Sparks
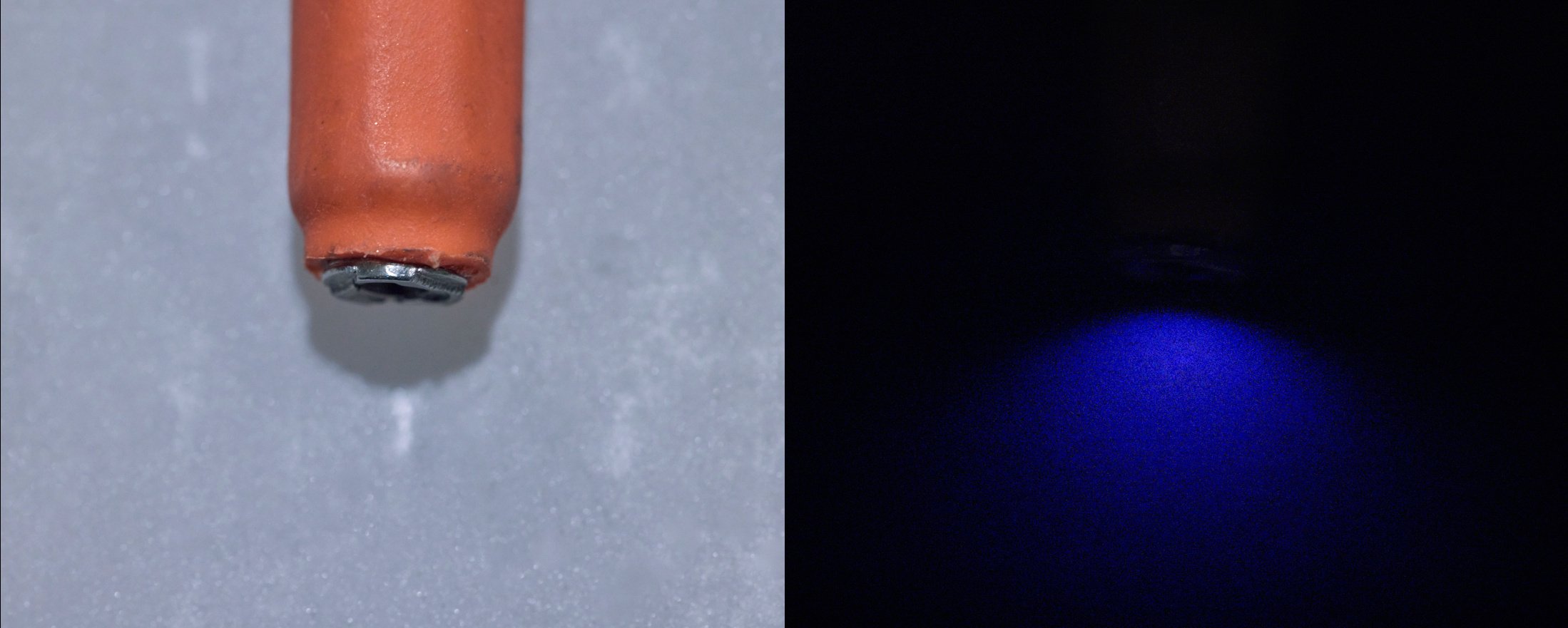
To see the scintillations, complete darkness is required. The human eye needs several minutes to adapt to darkness, during which the pupil dilates and rhodopsin accumulates in the retina. Once adapted, the faint glow around the source becomes visible. With the magnifying glass, this glow resolves into distinct, brief flashes of light—each one corresponding to a single atomic decay event.
The effect is mesmerizing: a constant, random roiling of sparks, like a miniature galaxy in motion. Each spark represents the violent disintegration of an atom, the release of nuclear binding energy, and the conversion of that energy into visible light. The randomness is Poissonian—decays occur at random intervals, but the average rate is fixed by the half-life of the isotope.
Technical Considerations and Safety
Alpha Particle Range
Alpha particles have limited penetration. In air, they travel only 2-5 cm before losing their energy. In solids, they penetrate mere micrometers. This means:
- The source must be very close to the screen (1-5 mm)
- The screen must be thin enough for alpha particles to reach the active ZnS layer
- The setup is inherently safe for external exposure since alpha particles cannot penetrate skin
Scintillator Efficiency
ZnS(Ag) has an excellent scintillation efficiency for alpha particles—approximately 30% of the particle's energy is converted to light. However, the light output is still very low:
- Each alpha particle produces ~2000-4000 photons
- The eye's dark-adapted threshold is about 100 photons per second entering the pupil
- With magnification, you can just barely exceed this threshold
Activity and Count Rate
A 37 kBq source (37,000 decays per second) seems like a lot, but geometry reduces the effective count rate:
- Only a fraction of decays produce alpha particles that hit the screen
- The solid angle subtended by the screen is small
- Typical observed count rates are 10-100 sparks per second
Building Your Own Spinthariscope
Materials Needed
- Alpha source: Americium-241 from a smoke detector (remove carefully, avoid damaging the foil)
- Scintillator: ZnS(Ag) coated plastic or glass. Search eBay for "spinthariscope screen" or "ZnS alpha detector"
- Magnifying glass: 5x to 10x magnification
- Dark environment: A light-tight box or a room with blackout curtains
- Mounting materials: Glue, stick, or holder for the source
Assembly Steps
- Extract the source: Carefully open a smoke detector and remove the small metal disc containing Americium-241. The activity is typically 0.9-1 µCi (37-40 kBq).
- Prepare the screen: If using a commercial ZnS screen, handle it gently—the coating is fragile.
- Mount the source: Glue it to a stick or holder for easy positioning.
- Dark adaptation: Spend at least 10 minutes in complete darkness before observing.
- Positioning: Place the source 2-5 mm from the screen, with the magnifying glass between your eye and the screen.
Alternative Sources
- Radium paint: Very old watch hands or instruments may have radium-226 paint. Handle with extreme caution—radium is more hazardous than Americium.
- Uranium ore: Surface-mineralized specimens (like autunite or torbernite) emit alpha particles. The activity is lower, so you may need a larger piece.
- Polonium-210: Extremely dangerous and not recommended for amateur use.
The Physics Behind the Sparks
Each spark represents a complete energy conversion chain:
- Nuclear decay: An Americium-241 nucleus decays via alpha emission to Neptunium-237, releasing ~5.5 MeV of energy.
- Energy transfer: The alpha particle collides with ZnS(Ag) atoms, exciting electrons.
- Scintillation: Excited electrons return to ground state, emitting photons (~450 nm wavelength).
- Photon collection: The magnifying glass concentrates photons into the eye.
- Visual perception: Photons trigger retinal cells, creating a perceived flash.
The randomness of the process is fundamental to quantum mechanics. Each decay event is independent and unpredictable, governed by the probabilistic nature of quantum tunneling through the nuclear potential barrier.
Comparison with Other Detection Methods
| Method | Sensitivity | Complexity | Cost | Educational Value |
|---|---|---|---|---|
| Spinthariscope | Low (single particles) | Very low | $20-60 | Excellent |
| Geiger-Müller counter | Medium (count rate) | Low | $100-300 | Good |
| Scintillation detector | High (energy resolution) | Medium | $500-2000 | Good |
| Semiconductor detector | Very high (energy resolution) | High | $2000+ | Specialized |
The spinthariscope offers unique educational value because it provides direct visual evidence of atomic processes. While a Geiger counter gives you a count rate, the spinthariscope shows you the individual events.
Safety Considerations
While the setup is generally safe, proper precautions are essential:
- Source handling: Never ingest, inhale, or create dust from the source. Use gloves and work in a clean area.
- Storage: Keep the source in a sealed container when not in use. Label it clearly.
- Disposal: Do not throw the source in regular trash. Contact local hazardous waste facilities for proper disposal.
- Children: This experiment should be supervised by adults. The educational value is high, but safety comes first.
Advanced Experiments
Once you've mastered the basic observation, you can explore:
- Distance dependence: Measure how the spark rate changes with distance. The range of alpha particles in air follows the Bragg curve.
- Shielding experiments: Try different materials between source and screen. Paper will stop alphas, but aluminum foil might not.
- Geometry effects: Change the angle between source and screen. The solid angle affects the count rate.
- Comparison with beta sources: If you have access to a beta source (like Strontium-90), compare the scintillation patterns. Beta particles produce different light patterns.
The Historical Context
The spinthariscope was invented by William Crookes in 1903, shortly after the discovery of radioactivity by Henri Becquerel. Crookes used radium as his source and zinc sulfide as his scintillator. His original design is remarkably similar to the modern version. The instrument played a crucial role in early nuclear physics, helping scientists understand the nature of radioactive decay.
Conclusion: A Window into the Atomic World
The spinthariscope provides a unique bridge between the macroscopic world we inhabit and the quantum world of atomic nuclei. Each spark is a direct visualization of a fundamental process that powers stars, enables radiometric dating, and poses both risks and benefits in medicine and industry.
For homelab builders and hardware enthusiasts, this experiment offers a different kind of benchmarking—not of CPU cycles or storage IOPS, but of nuclear decay rates and scintillation efficiency. It's a reminder that the most fascinating phenomena often occur at the extremes of scale, where individual atoms become visible through their violent disintegration.
The equipment needed is minimal, the physics is profound, and the experience is unforgettable. In an age of digital displays and virtual reality, there's something uniquely compelling about seeing the raw energy of atomic decay with your own eyes, unmediated by electronics or software.
Resources and Further Reading
- NIST Nuclear Physics Reference Data
- Oak Ridge Associated Universities - Americium-241
- ZnS(Ag) Scintillator Properties
- [Historical paper: Crookes, W. (1903). "On the Radioactivity of Uranium and other Minerals." Philosophical Transactions of the Royal Society A]
For those interested in purchasing pre-made spinthariscopes, search for "spinthariscope" on scientific equipment suppliers or eBay. Prices typically range from $40 to $80 for a complete unit with a safe, low-activity source.

Comments
Please log in or register to join the discussion